Research Article - Der Pharma Chemica ( 2021) Volume 13, Issue 11
New Route for the synthesis of Bosutinib
Pawan Kumar*, Mursyidah Rahim, Suhaila M. Yaman and Sandeep MhetrePawan Kumar, Department of Chemical Research and Development, Oncogen Pharma, Malaysia, Email: pawan.kumar@oncogenpharma.com
Abstract
An improved and efficient synthesis route to bosutinib is reported. The initial route introduced by Pfizer, implemented Gould-Jacob reactions as key steps. In this protocol, the synthesis, isolation characterization of novel intermediates and their application in the alkylation step for the synthesis of bosutinib are briefly described, using an economic and practical procedure. Ultimately, we provide a new alternative of bosutinib synthesis route, through alkylation of alkali-intermediate with good yield.
Keywords
Bosutinib, Alkali metal salts, N-Alkylation, Novel intermediates, Improved protocol
Introduction
In the past three decades targeted therapy have been a major interest in the pharmaceutical research [1]. This approach is not limited to aim onto the target cells, but it offers minimal interaction towards non-target tissues [2]. One of the target-oriented drugs is tyrosine kinase inhibitor.
Numerous clinically proven tyrosine kinase inhibitors have been studied in the treatment of chronic myeloid leukaemia (CML). Some of the common molecules used in this treatment are bosutinib, imatinib, Dasatinib and nilotinib [3].
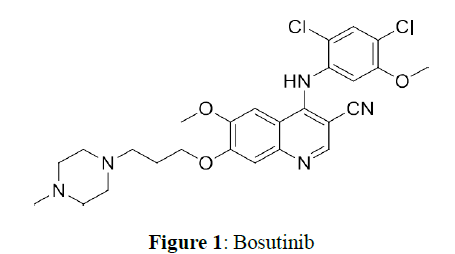
Bosutinib 1, chemically 4-((2,4-dichloro-5-methoxyphenyl) amino)-6-methoxy-7-(3-(4- methylpiperazin-1-yl) propoxy) quinoline-3-carbonitrile is an ATP-competitive Bcr-Abl tyrosine kinase inhibitor with an additional inhibitory effect on Src family kinase for use in the treatment of cancer. Bosutinib has received approval of the USFDA for the treatment of adult patients with Philadelphia chromosome-positive (Ph+) chronic myelogenous leukaemia (CML) with resistance or intolerance to previous therapy (Figure 1).
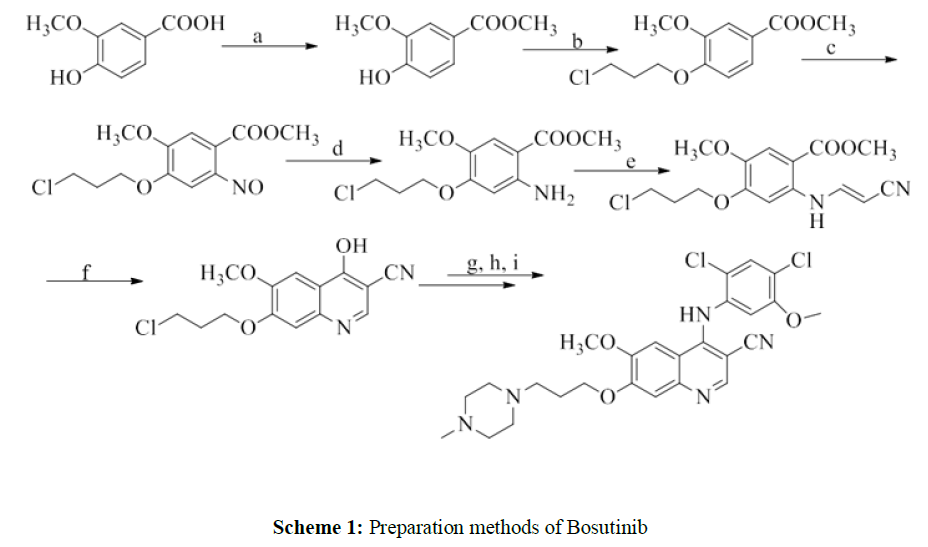
Preparation methods of Bosutinib have been extensively explored on milligram scale. Gould- Jacob method was commonly employed in the previous works to prepare Bosutinib [4,5]. For instance, [6] started the process with alkylation of the hydroxyl group of methyl 3-hydroxy-4- methylbenzoate. The process is limited by its yield (8.3%) and reaction temperature (-78°C). Later, [7] improvised this method by introducing nitro group into compound methyl 3-hydroxy-4- methylbenzoate as depicted in Scheme 1. They improved the overall yield to 21.7% and managed to avoid use of extreme reaction temperature. However, these methods involved complex and sequential reactions.
Reagents: a) methanol; b) 1-bromo-3-chloropropane; c) HNO3; d) Fe/NH4Cl; e) 3,3- diethoxypropionitrile, trifluoroacetic acid; f) NaOH; g) POCl3; h) 2,4-dichloro-5-methoxyaniline, pyridine hydrochloride; i) NaI, N-methylpiperazine.
Scheme 1 Synthesis route by [7]
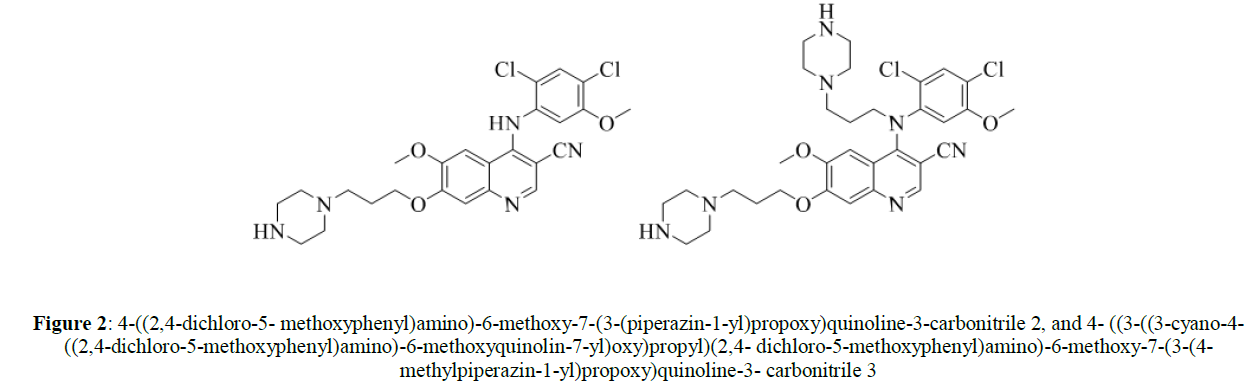
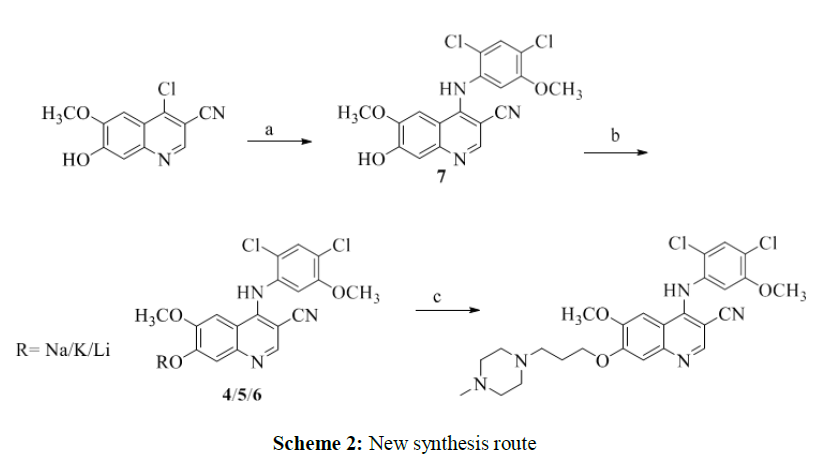
A considerable works describing formation of impurities such as 4-((2,4-dichloro-5- methoxyphenyl)amino)-6-methoxy-7-(3-(piperazin-1-yl)propoxy)quinoline-3-carbonitrile 2, and 4- ((3-((3-cyano-4-((2,4-dichloro-5-methoxyphenyl)amino)-6-methoxyquinolin-7-yl)oxy)propyl)(2,4- dichloro-5-methoxyphenyl)amino)-6-methoxy-7-(3-(4-methylpiperazin-1-yl)propoxy)quinoline-3- carbonitrile 3, as per Scheme 2, reported hurdle in removing these impurities hence extensive purification technique is required. In addition, use of corrosive reagents and high boiling solvent such as concentrated nitric acid and DMF are other drawbacks in these processes (Figure 2).
Figure 2: 4-((2,4-dichloro-5- methoxyphenyl)amino)-6-methoxy-7-(3-(piperazin-1-yl)propoxy)quinoline-3-carbonitrile 2, and 4- ((3-((3-cyano-4-((2,4-dichloro-5-methoxyphenyl)amino)-6-methoxyquinolin-7-yl)oxy)propyl)(2,4- dichloro-5-methoxyphenyl)amino)-6-methoxy-7-(3-(4-methylpiperazin-1-yl)propoxy)quinoline-3- carbonitrile 3
To avoid tedious preparation steps and overcome these impurities and yield problems, there is need to develop a facile, economic, convenient, and commercially viable process. Thus, we have developed an improved route for the synthesis of bosutinib, by isolating the alkali metal salts 4-6 of the phenolate anion of compound 7.
Materials and Methods
General
Starting material 4-chloro-7-hydroxy-6-methoxyquinoline-3-carbonitrile, 2,4-dichloro-5- methoxyaniline and 1-(3-chloropropyl)-4-methylpiperazine dihydrochloride were procured from manufacturing companies in China and used as such without purification. All the general chemicals were brought from Merck Sdn. Bhd., Malaysia. Solvents were from Polyscientific Enterprise Sdn. Bhd., Malaysia. 1H NMR and 13C NMR were recorded in DMSO-d6 at 300 and 75 MHz, respectively using Bruker instrument. All the chemical shift values are reported in δ units downfield from TMS as internal standard. X-Ray diffraction pattern (XRD) analysis was performed using PANalytical instrument model Empyrean.
Melting points were recorded using BUCHI melting point apparatus.
Preparation of 4-((2,4-dichloro-5-methoxyphenyl) amino)-7-hydroxy-6-methoxyquinoline-3- carbonitrile (7)
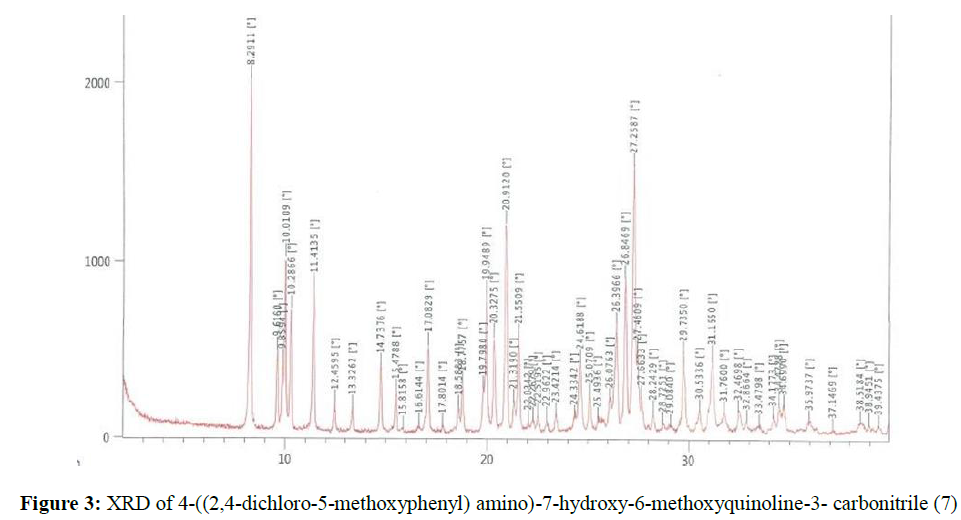
2,4-dichloro-5-methoxyaniline (23.53 g, 0.1225 mol) was added to a suspension of 4-chloro-7- hydroxy-6-methoxyquinoline-3-carbonitrile (25.0 g, 0.1065 mol) in acetonitrile (125 ml). Hydrochloric acid in isopropyl alcohol (12.5 ml) was added dropwise into the resulting mass. The resulting mass was stirred and 75-80ºC and progress of the reaction was monitored through TLC. The reaction was completed in 2-3 h. The reaction mass was filtered and the solid was washed with acetonitrile then suck dried. (36.7 g, 88.3%). 1H NMR (300 MHZ, DMSO-d6) δ 3.91 (s, 3H), 4.05 (s, 3H), 7.58 (s, 1H), 7.64 (s, 1H), 7.80 (s, 1H), 8.41 (s, 1H), 8.99 (s, 1H), 11.67 (s, 1H) ppm. 13C NMR (75 MHZ, DMSO-d6) δ 56.91, 57.03, 84.93, 103.51, 104.26, 111.08, 113.63, 114.5, 115.6, 122.3,123.8, 129.73, 134.04, 134.87, 142.95, 146.12, 150.38, 153.94 ppm. The X-ray powder diffractogram peaks were at 8.29, 9.62, 9.85, 10.01, 10.29, 11.41, 14.74, 15.48, 17.08, 18.78, 19.80, 19.95, 20.33, 20.91, 21.55, 24.62, 25.07, 26.40, 26.85, 27.26, 27.46, 27.66, 29.74, 31.16 ± 0.2 [°theta], Figure 3. A sharp melting point was not observed, but solid discoloration with decomposition of the compound was observed within the range of 224.9 – 235.8ºC.
Preparation of sodium 3-cyano-4-((2,4-dichloro-5-methoxyphenyl) amino)-6-methoxyquinolin- 7-olate (4)
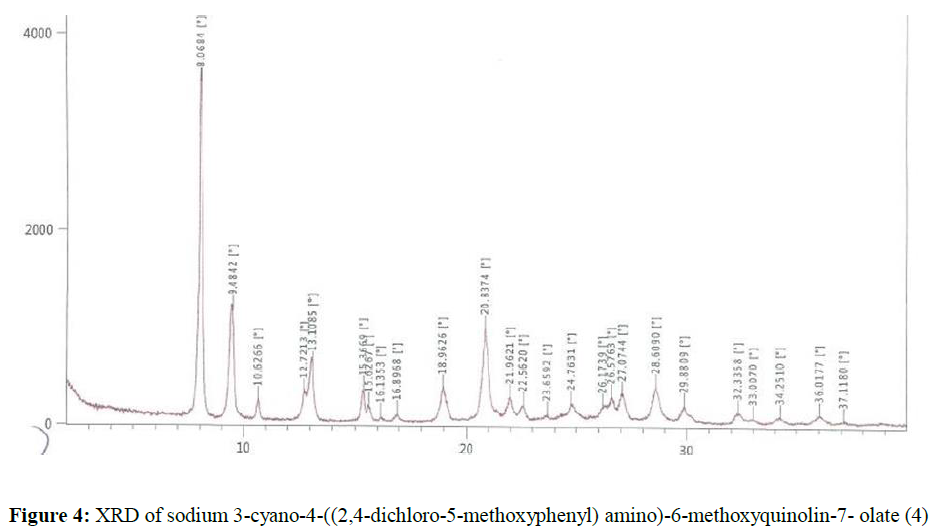
Sodium hydroxide (6.0 g, 0.15 mol) was dissolved in methanol (250 ml). Then it was added into a solution of 4-((2,4-dichloro-5-methoxyphenyl) amino)-7-hydroxy-6-methoxyquinoline-3- carbonitrile, 7 (50.0 g, 0.1281 mol) in methanol (750 ml). The reaction mass was stirred at 60-65ºC for 1 h. The obtained solid was filtered and washed with methanol. The wet solid was dried under vacuum at 60-65ºC to get the titled compound 4 (44.9g). The X-ray powder diffractogram peaks were at 8.07, 9.48, 13.11, 20.84, 28.61 ± 0.2 [°theta] Figure 4. A sharp melting point was not observed, but solid discoloration with decomposition of the compound was observed within the range of 245.4 - 262.8ºC.
Preparation of lithium 3-cyano-4-((2,4-dichloro-5-methoxyphenyl) amino)-6-methoxyquinolin- 7-olate (5)
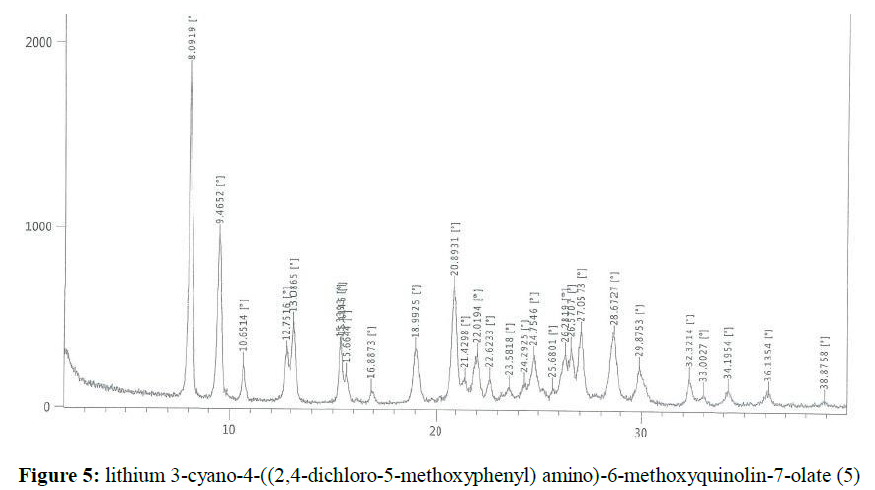
Lithium hydroxide (0.828 g, 0.02353 mol) was dissolved in methanol (50 ml). Then it was added into a solution of 4-((2,4-dichloro-5-methoxyphenyl) amino)-7-hydroxy-6-methoxyquinoline-3- carbonitrile (10.0 g, 0.0256 mol) in methanol (150 ml). The reaction mass was stirred at 60-65°C for 1 h. The obtained solid was filtered and washed with methanol. The wet solid was dried under vacuum at 50-55°C to get the titled compound 5 (7.59g). The X-ray powder diffractogram peaks were at 8.09, 9.47, 10.65, 12.75, 13.09, 15.34, 15.41, 22.02, 24.75, 26.28, 26.57, 27.06, 28.67, 29.88 ± 0.2 x[°theta], (Figure 5). A sharp melting point was not observed, but solid discoloration with decomposition of the compound was observed within the range of 223.8– 248.8ºC.
Preparation of potassium 3-cyano-4-((2,4-dichloro-5-methoxyphenyl) amino)-6- methoxyquinolin-7-olate (6)
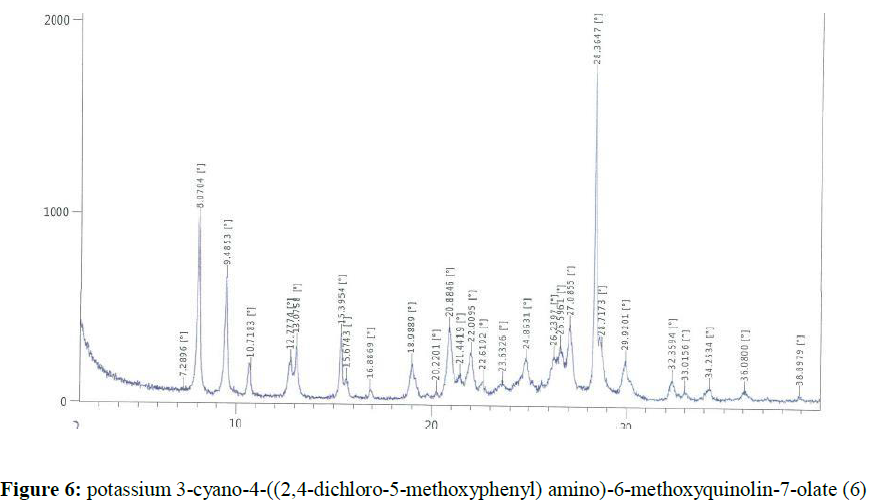
Potassium hydroxide (1.65 g, 0.029mol) was dissolved in methanol (50 ml). Then it was added into a solution of 4-((2,4-dichloro-5-methoxyphenyl) amino)-7-hydroxy-6-methoxyquinoline-3- carbonitrile (10.0 g, 0.0256 mol) in methanol (175 ml). The reaction mass was stirred at 60-65°C for 1 h. The obtained solid was filtered and washed with methanol. The wet solid was dried under vacuum at 50-55°C to get the titled compound 6 (8.23g). The X-ray powder diffractogram peaks were at 8.07, 9.49, 12.78, 13.08, 15.40, 18.99, 20.88, 22.01, 24.86, 26.24, 26.60, 27.09, 28.36, 28.72, 29.92 ± 0.2 [°theta], (Figure 6). A sharp melting point was not observed, but solid discoloration with decomposition of the compound was observed within the range of 239.3– 260.7°C.
Preparation of 4-((2,4-dichloro-5-methoxyphenyl) amino)-6-methoxy-7-(3-(4-methylpiperazin- 1-yl) propoxy) quinoline-3-carbonitrile (1)
Sodium 3-cyano-4-((2,4-dichloro-5-methoxyphenyl) amino)-6-methoxyquinolin-7-olate 4 (50 g, 0.1213 mol) was added into solution of triethylamine (30.5 ml) and 1-(3-chloropropyl)-4- methylpiperazine dihydrochloride (31.79 g, 0.1274 mol) in acetonitrile (535 ml). The reaction mass was stirred at 75-80 °C overnight. The reaction mass was cooled to room temperature then the solvent was distilled off, slurried the compound in methanol, filtered and dried to afford compound 1.
Results and Discussion
The initial studies were based on the isolation and stability of all the alkali metal salts of compound 7 and their impact on the O-alkylation reaction. The synthesis of these salts was screened based on several parameters such as their molar equivalent, reaction time and reaction temperature. Required molar equivalent was studied ranging from 1.0 molar equivalent to 1.2 molar equivalent, and 1.1 molar equivalent was finalized in resulting optimum conversion of the salts.
Next, selecting alkali metal hydroxide was challenging due to effectiveness of each salt compound in O-alkylation. Sodium salt was preferred since it afforded best yield among the salts. Low-boiling solvents such as methanol, iso-propanol, acetonitrile, dichloromethane, and n- hexane were screened to replace utilization of high-boiling solvents. Among these solvents, acetonitrile was selected since O-alkylation was hindered in polar protic solvent.
We reported synthesis of bosutinib in three steps as per Scheme 2, starting from N-alkylation of 2,4-dichloro-5-methoxyaniline by 4-chloro-7-hydroxy-6-methoxyquinoline-3-carbonitrile which afforded 4-((2,4-dichloro-5-methoxyphenyl) amino)-7-hydroxy-6-methoxyquinoline-3-carbonitrile 7. Then, alkali metal salts of formula 4, 5 and 6 were isolated from compound 7. Lastly, bosutinib was obtained from alkylation of the corresponding alkali metal salts by 1-(3-chloropropyl)-4- methylpiperazine dihydrochloride (Scheme 2).
Reagents and conditions: (a) 2,4-dichloro-5-methoxyaniline, acetonitrile, HCl in isopropyl alcohol, 75-80ºC; (b) NaOH/ LiOH.H2O/KOH, methanol, 60-65 °C; (c) 1-(3-chloropropyl)-4- methylpiperazine dihydrochloride, acetonitrile, 75-80ºC.
Conclusion
A new and convenient synthesis route of bosutinib with isolable and stable intermediate was developed. The intermediates were stable and manageable from milligram to multigram scale. Moreover, this method avoided use of high boiling solvent which is highly advantageous in terms of scale-up and yield.
References
- Eder J, Sedrani R and Wiesmann C. Nat Rev Drug Disc. 2014, 13(8): p. 577.
- Abouzid K and Showman S. Bioorg Med Chem. 2008, 16: p. 7543.
- Kimura S. Springer, Singapore, 2017, 55.
- Mao Y, Zhu C, Kong Z et al., Ren Synthesis. 2015, 47(20): p. 3133.
- Zhang N, Wu B, Powell D et al., Bioorg Med Chem Lett. 2000, 10(24): p. 2825.
- Boschelli DH, Ye, Wang YD et al., J Med Chem. 2001, 44(23): p. 3965.
- Yin XJ, Xu GH, Sun X et al., Molecules. 2010, 15(6): p. 4261.