Research Article - Der Pharma Chemica ( 2018) Volume 10, Issue 11
Plant Extract Mediated AgNps Synthesis in Averrhoa bilimbi and Syzygium cumini Leaf Extract, their Characterization and Antioxidant Potential Assessment
Vijay D Mendhulkar*, Sopan N Kharat and Snehal R Mahadik
Department of Botany, The Institute of Science, 15, Madame Cama Road Mumbai, Maharashtra-400032, India
- *Corresponding Author:
- Vijay D Mendhulkar
Department of Botany
The Institute of Science
15, Madame Cama Road Mumbai, Maharashtra-400032, India
Abstract
The approach of green synthesis of silver nanoparticles was focused in the present work using leaf extract of two medicinal plants, Averrhoa bilimbi L. and Syzygium cumini L. in different intensities of sunlight. The method is light sensitive, simple, eco-friendly and quick for the synthesis of AgNPs from leaf extracts. These plants extract mediated SNP’s were comparatively characterized and evaluated for their antioxidant properties independently. The synthesized AgNPs were characterized by UV-spectroscopy, Nanoparticle Tracking Analysis (NTA), X-ray Diffraction (XRD), Transmission Electron Microscopy (TEM) and Fourier Transformed Infrared spectrum (FTIR) analysis. The UV-spectra showed maximum absorbance at 430 nm, NTA analysis shows 20 nm average sizes of AgNPs synthesized in A. bilimbi and 50 nm size in S. cumini leaf extract respectively. XRD analysis showed four Bragg’s reflection planes of the metallic silver in the 2θ range of 30º-80º and indexed as (111), (200), (220) and (311). TEM analysis revealed spherical shape of synthesized AgNPs in both the samples with similar diameter as recorded in NTA analysis. FTIR spectra indicates characteristic of the hydroxyl functional group in alcohols and phenolic compounds. The radical scavenging activity of silver nanoparticle was evaluated using DPPH assay. AgNPs samples of A. bilimbi and S. cumini showed notable scavenging potential indicating the ability of plant extract mediated AgNPs as a free radical scavenger.
Keywords
Averrhoa bilimbi, Syzygium cumini, NTA, XRD, TEM, FTIR, UV-visible, Antioxidant.
Introduction
Synthesis of nanoparticles is an appealing field for researcher due to its wide scope in various fields like physics, chemistry, electrical engineering, material sciences and in life sciences especially for bio-medical application. The plant’s having its own potentials to produce secondary metabolites as a defense mechanism. Biological syntheses of nanoparticles can easily be scaled up in short duration for its commercialization. Production of natural products in nanoscale form but in bulk amount is a need of hours to cure various diseases. Nanoparticles exhibit unique properties due to their size, distribution and morphology [1]. The Silver nanoparticles are nontoxic, safe inorganic antibacterial agent. They are used for the cure of over 500 types of diseases caused by micro-organisms [2]. Nanoparticles can be synthesized by chemical, green and microbial methods [3]. The method available for green synthesis of silver nanoparticles is more effective, reliable, rapid and efficient. Recently, nanoparticles are attracting universal attention due to their unique physical and chemical properties and are used for various applications in medicine, biology and biotechnology [4].
The antioxidant, antibacterial and antifungal properties of silver nanoparticles have been studied by many workers. Their published reports indicate that nano silver drugs are a promising and special class of biocidal agents [5]. Plants are the potential source of natural antioxidants. Natural antioxidants or phytochemical antioxidants are the secondary metabolites of plants. The production of silver nanoparticles from plant extract based protocol fulfills all the criteria for green synthesis and it is suitable for large scale productions. They are safe for human therapeutic use [6].
Averrhoa bilimbi Linn., a member of family Oxalidiaceae, is an attractive, long lived tree grows 5-10 m in height. The plant is native from Indonesia and Malaysia. The plant is having worldwide distribution like Australia, Brazil, Bangladesh, India, Philippines, Myanmar, Malaysia, Sri Lanka, Thailand and Zanzibar. Leaves are clustered at the branch tips, alternate with 11-37 sub-opposite leaflets. The fruits are good source of antioxidants and traditionally the leaves are used as paste on itches, swelling, rheumatism, mumps or skin eruptions, after-birth tonic and also in cold and cough, bites of poisonous creatures, etc [7]. A leaf decoction is taken to relieve rectal inflammation. The extract obtained by decocting the leaves of A. carambola is used for the treatment of diabetes. Fruits are used in inflammatory hepatitis, fever, diarrhea, bilious colic, mouth ulcers, toothache, nausea, ascites etc.
Syzygium cumini L. Skeels, is tree belongs and to the family Myrtaceae. This large evergreen tree is distributed throughout India and commonly known as ‘Jamun’. The plant is native to Bangladesh, India, Nepal, Pakistan, Sri Lanka, Philippines and Indonesia. Leaves are exstipulate, petiolate, simple, and elliptic to broadly oblong, flowers white. Ayurveda and Unani system of medication have mentioned variety of therapeutic properties of the plant as carminative, anthelmintic, analgesic, anti-inflammatory, antioxidant, as well as gastro protective agents. The plant having various medicinal properties and used in Ayurveda as a stomachic, astringent, antiscorbutic, diuretic, antidiabetic, and in chronic diarrhea and enlargement of spleen [8]. Previous studies have demonstrated that S. cumini essential oil has antioxidant [9] antibacterial and antifungal activity [10]. S. cumini essential oil of Indian origin is not only a source for novel calamenene type sesquiterpenoids but also possesses good anti-fungal potential. S. cumini shows high antioxidant activity. This activity was observed when an increase in levels of plasma glucose, vitamin -E, ceruloplasmin, lipid peroxides and a decrease in levels of vitamin C and glutathione [11].
The literature survey on these plant systems indicates scanty reports on the synthesis of silver nanoparticles from leaf extract. Hence, the present study is undertaken to phytosynthesized and characterizes the AgSNP’s in A. bilimbi and S. cumini and to evaluate their antioxidant potentials.
Materials and Methods
Plant material and chemicals
The fresh and healthy leaves of the experimental plant A. bilimbi and S. cumini were collected from the campus of The Institute of Science, Mumbai, in December, 2015. Both the plants were taxonomically authenticated with Blatter herbarium, Mumbai. Leaves were rinsed thoroughly with the tap water followed by distilled water to remove the dust, pathogens and adhered matter. Silver nitrate (AgNO3) was procured from the Merck and DPPH (1,1-Diphenyl-2-picrylhydrazyl) from Sigma Aldrich (Figure 1).
Preparation of plant extracts
Leaves weighing 10 g were thoroughly washed in distilled water for 5 min, dried, cut into fine pieces and were boiled in a conical flask with 100 ml of double distilled water up to 15 min and filtered through Whatmann No. 1 filter paper. The extract was filtered twice to obtained clear solution.
Sunlight mediated synthesis of silver nanoparticles in plant extract
For the synthesis of AgNPs, 50 ml of aqueous plant leaf extract and 50 ml 10 mM silver nitrate (AgNO3) solution was mixed with 400 ml deionized water in a conical flask. Quickly the mixture was placed in sunlight. A change in the color was observed indicating the beginning of the formation of silver nanoparticle. The color change of the mixture reddish to colloidal was monitored periodically using Uv-Visible spectrophotometer. After the synthesis and completion of reaction the solution was centrifuged at 10,000 rpm for 20 min. The transparent solution was discarded and the pellets of silver nanoparticles were collected. The pellets were dried in the oven at 45°C to 50°C.
UV-visible spectra analysis
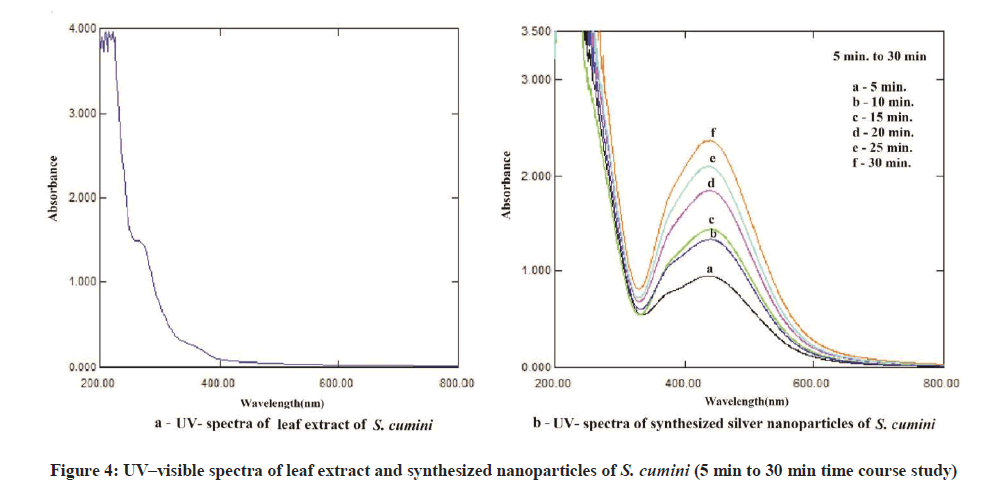
The bioreduction of Ag+ ions in each sample was observed periodically followed by the dilution with 2 ml of double distilled water. This AgNPs synthesized mixture was scan in the range of 200 to 800 nm wavelengths under UV–visible spectrophotometer (Model-Shimadzu UV 1800, Germany). The spectra were recorded at the intervals of 1 min to 10 min. The distilled water was used as a baseline. The silver nanoparticles are commonly characterized using UV-Vis spectroscopy, which is one of the most widely used techniques for the structural characterization of silver nanoparticles.
Characterization of silver nanoparticles (AgNPs)
Nanoparticles tracking analysis
The SNPs were visualized using Nanoparticle tracking analysis (NTA- Nanosight UK-LM20). The technique is used in conjunction with an ultra microscope and a laser illumination unit that together allow small particles in liquid suspension to be visualized moving under Brownian motion. The light scattered by the particles is captured using a CCD camera over multiple frames. Analysis of particles at the lowest end in the range of 10 to 1000 nm is possible only for particles composed of materials with a high refractive index, such as gold and silver. The pinch of silver nanoparticles was taken in 1 ml distilled water and sonicated to make the suspension. The samples were injected by sterile syringe.
X-ray diffraction (XRD) analysis
1 mg of nanoparticles powder was dissolved in 1 ml of distilled water in eppendorf tube to obtain a thick film. The dry powder of the silver nanoparticles was used for XRD analysis. The diffraction pattern was recorded from diffraction angle range of 20º to 80º. The XRD method is suitable to determine the crystal structures by analyzing the positions and intensities of diffraction peaks typically observed for well-crystallized material. The diffraction pattern corresponds to the pure silver metal powder. The XRD studies were conducted using Rigaku miniflex bench top X-ray spectrophotometer. The X-rays are generated using cathode ray tube, filtered to produce monochromatic radiation, collimated to concentrate, and directed toward the sample. X-ray diffraction (XRD analysis) is a unique method in determination of crystallinity of a compound. XRD analysis is based on constructive interference of monochromatic X-rays and a crystalline sample.
Fourier transform infra-red (FTIR) spectroscopy
The AgNPs (1 mg) powder was taken in an eppendorf tube and the suspension was prepared in 1 ml distilled water. This suspension was sonicated. In clean, dry watch glass a potassium bromide (KBr) powder was taken and 3 to 4 drops of suspension was added to it. The mixture was grinded using mortar and pestle to make it fine powder. FTIR measurement was carried out to identify the group of biomolecules responsible for the surface capping and reducing the Ag salt. Fourier Transform Infrared Spectroscopy (FTIR) identifies chemical bonds in a molecule by producing an infrared absorption spectrum. FTIR is an effective analytical instrument for detecting functional groups and characterizing covalent bonding information. An FTIR spectrum was recorded using Perkin Elmer FTIR Spectrum-100 model.
Transmission electron microscopy (TEM)
Transmission electron microscopy (TEM) technique was used to visualize the morphology of the synthesized AgNPs. A drop of the SNPs colloidal solution was placed on carbon coated copper grids, later exposed to infrared light (30 min) for solvent evaporation. TEM analysis was performed on PHILIPS model CM 200, operated at an accelerating voltage of 200 kV with the resolution of 0.22 nm. An image is formed by the interaction of the electrons transmitted through the specimen. The image is magnified and focused onto an imaging device, such as a fluorescent screen, on a layer of photographic film, or to be detected by a sensor such as a CCD camera.
Antioxidant activity (DPPH radical scavenging assay)
DPPH scavenging activity of SNPs was measured using the method described by Brand-Williams et al., with slight modifications. DPPH (1,1-diphenyl-2-picrylhydrazyl) methanolic solution, (0.002%) was used in the present study. DPPH concentration is reduced by the existence of an antioxidant at 515 nm and the absorption gradually disappears with time. Briefly, 0.002% solution of DPPH in methanol was prepared and 180 μl of this solution was added to 20 μl of the solution of all AgNPs samples at different concentrations. 10 mg AgNPs and aqueous both plant extracts were dissolved in 1 ml of HPLC water to obtain the aliquot of AgNPs as well as plant extracts (50, 100, 150, 200 and 250 μg/ml). The diluted working solutions of the test AgNPs and plant extracts were prepared using HPLC water (sonicated for 10 min). The mixtures were kept at room temperature for 30 min. The analysis was performed using a Synergy H1-Multi plate reader (Biotek) instrument. Ascorbic acid was used as a positive control (standard) and methanol as a blank. The experiments were performed in triplicate and percentage scavenging activity was calculated using following equation.
[(Absorbance control–Absorbance sample)/Absorbance control] × 100
All the experiments were repeated in triplicate and means with standard deviation was calculated.
Statistical analysis
The scavenging percentage of all samples was determined. The DPPH assay experiment was performed in triplicates (n=3) and the final results were represented as % of DPPH free radical scavenging activity (μg/ml). Statistical analyses for IC50 were carried out using Microsoft excel 2007.
Results and Discussion
The present study deals with the green synthesis of silver nano-particles, their characterization and their evaluation for antioxidant activity. The aqueous extract was used to the synthesis of AgSNP’s from two medicinally important plants, A. bilimbi and S. cumini. Both the plant synthesized silver nanoparticles in bulk amount and showed antioxidant radical scavenging activities. The findings are discussed below.
UV–vis spectroscopy
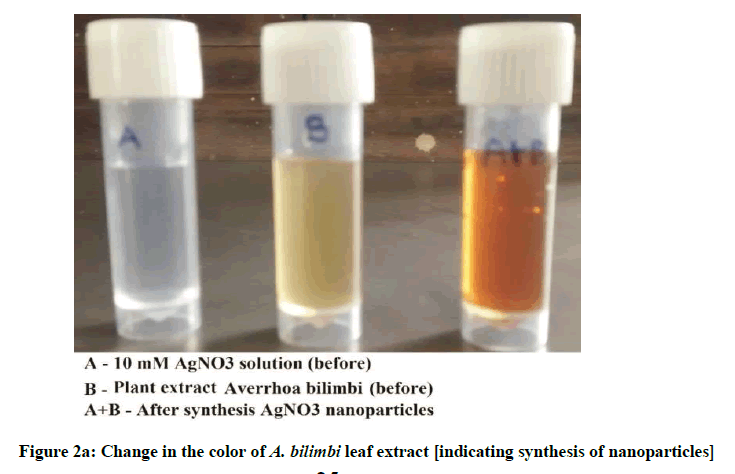
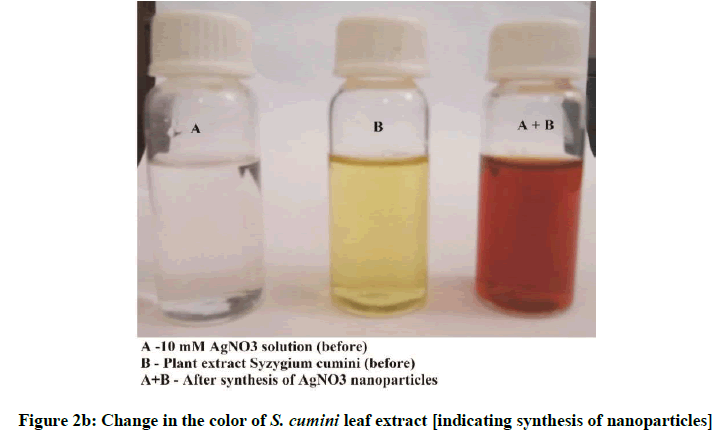
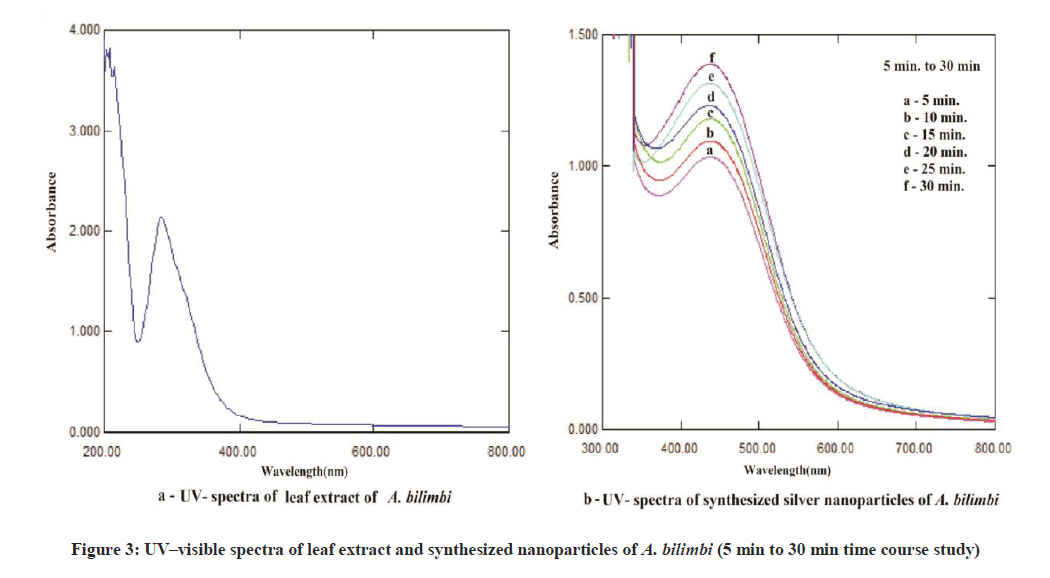
The color change showed the presence of Ag nanoparticles in the A. bilimbi and S. cumini leaf extracts and it was characterized by UV-Vis spectrophotometer and monitored by taking readings at regular time intervals in UV-vis spectrophotometer. The formation of silver nanoparticles following the addition of AgNO3 solutions was observed visually by a change in color of the solutions. The color change in AgNO3 solution appeared immediately within 1 min of addition of plant extract (Figures 2a and 2b). The UV-vis spectra for both the leaf extract samples was determined and it shows the stable absorbance at 320 nm for both the extract (Figures 3 and 4). The strong UV-Vis spectrum for the plant leaf extract reaction mixtures showed an absorption peak at 430 nm (Figures 3 and 4). The position of the absorption peak depends on the particle size and shape [12].
NTA analysis
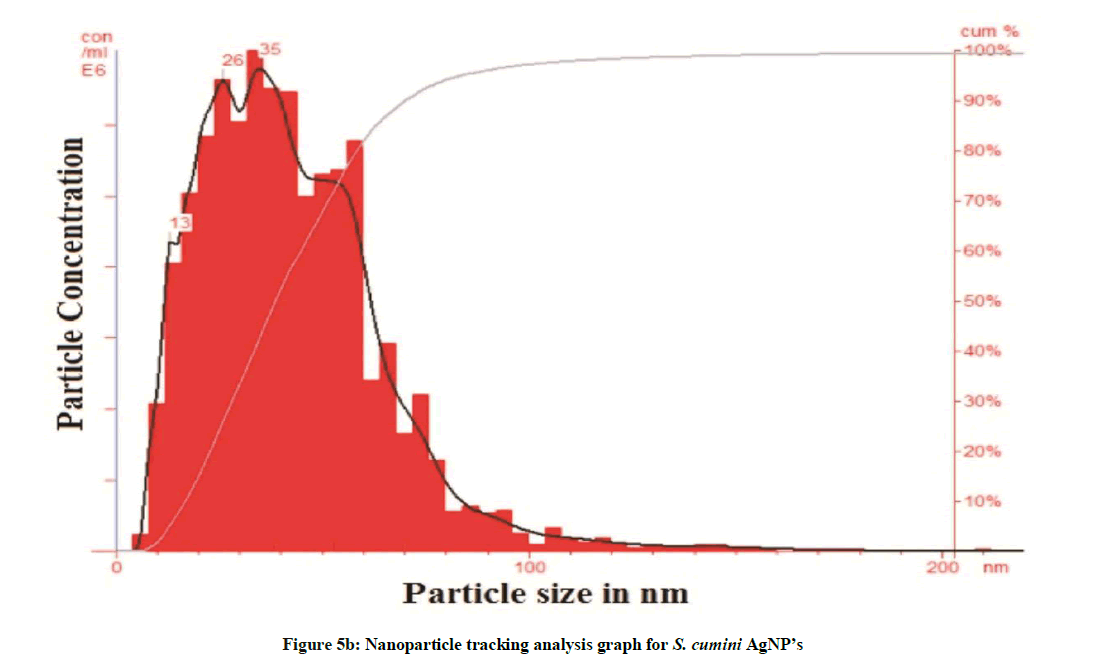
The particle size analyzer (NTA- LM-20) was used to determine the size of synthesized nanoparticles and the average particle size was recorded to be 36 nm for A. bilimbi and 44 nm S. cumini mediated AgNPs. The frequency of phytoreduced A. bilimbi nanoparticle as recorded by NTA is 61.30 particles / frame (2.73× 8 particles/mL) and for S. cumini it was 77.22 particles / frame (3.48 × 8 particles / ml). The size variation is evident in the histogram (Figures 5a and 5b). The findings of NTA analysis were confirmed by TEM analysis. Kashid et al. [13] reported antibody tagged gold nanoparticles average size 20 nm using NTA analysis.
Transmission electron microscopy (TEM) analysis
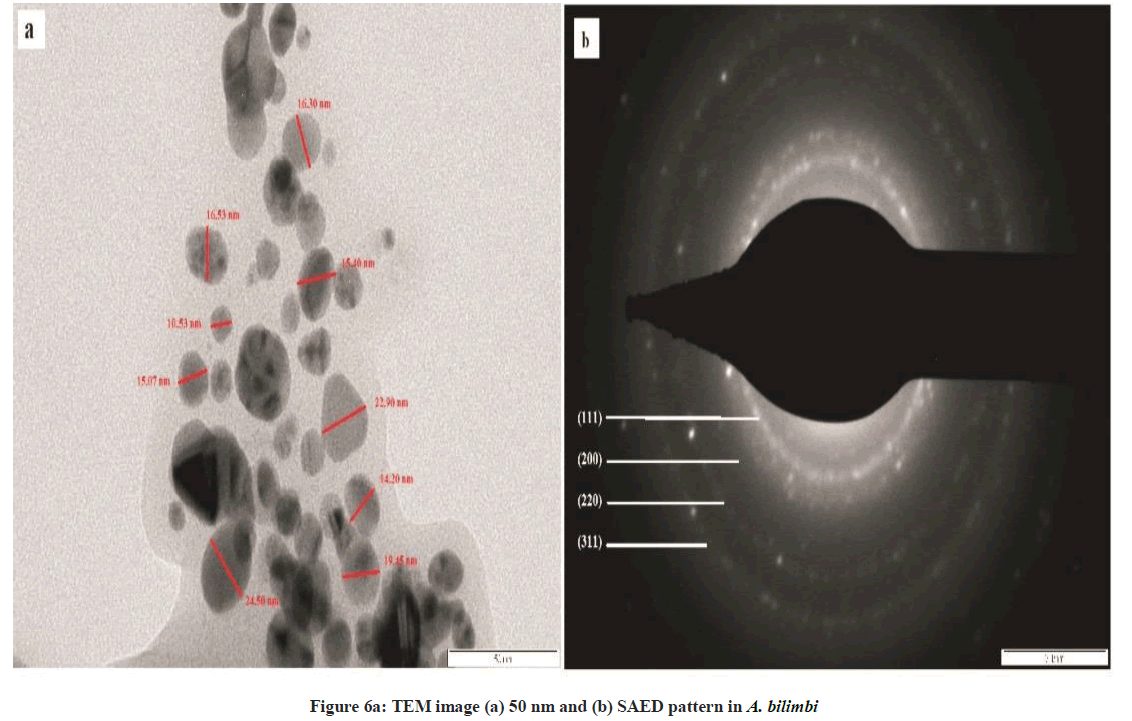
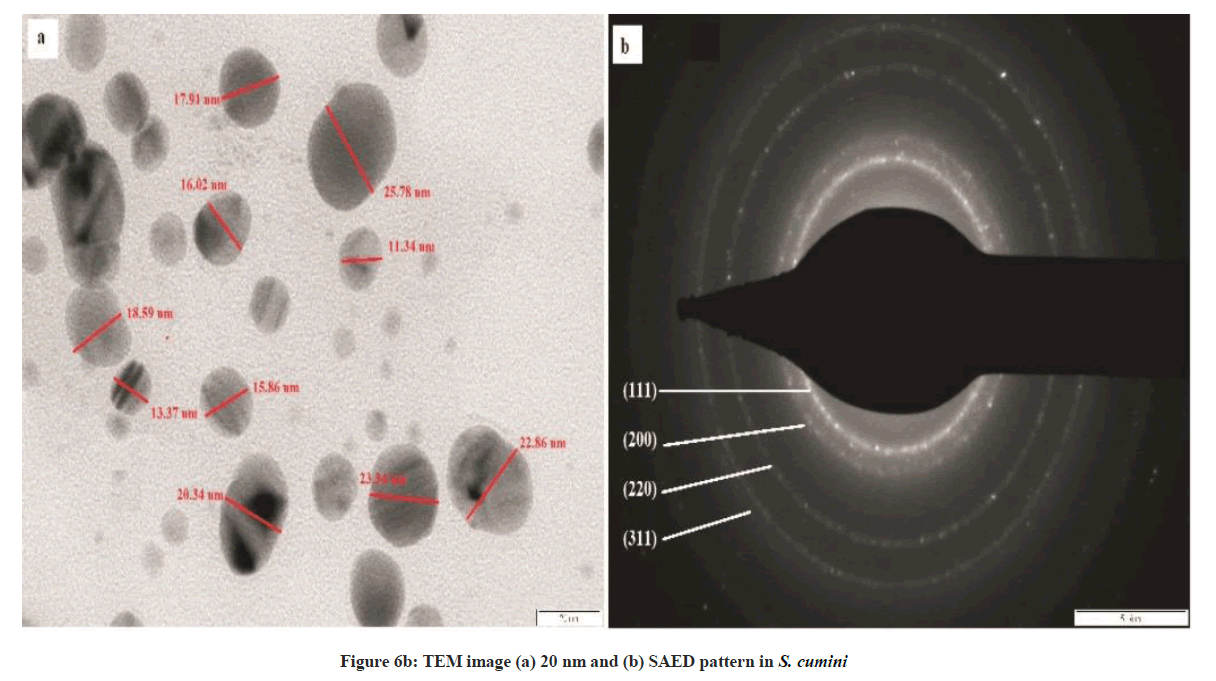
The synthesized AgNPs were spherical in shape as reflected by TEM analysis (Figures 6a and 6b). The peak produced by AgNPs in UV-visible spectroscopy and the particle size as determined by NTA analysis was confirmed by TEM technique. The TEM images revealed that the synthesized AgNPs are spherical in structure (Figure 6a). Selected area electron diffraction (SAED) pattern obtained from an AgNPs showed the diffraction rings from inner to outer associated with the (111), (200), (220), and (311) atomic planes of Ag indicating the formation of crystalline silver nanoparticles. As per TEM analysis, the average diameter of AgNP’s particles was 50 nm for A. bilimbi mediated SNPs and 20 nm for S. cumini. Many researchers have reported that the particle size is illicit by the leaf extract [12,14]. Santhoshkumar et al. [15] reported in Cinnamomum camphora the silver nanoparticles are spherical, circular and triangle in shape.
X-ray diffraction profile
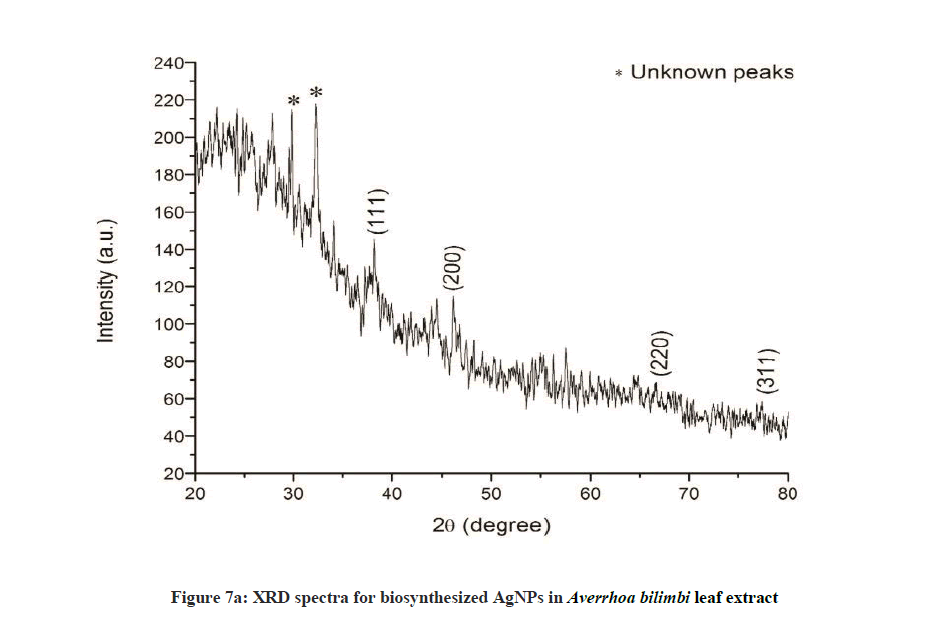
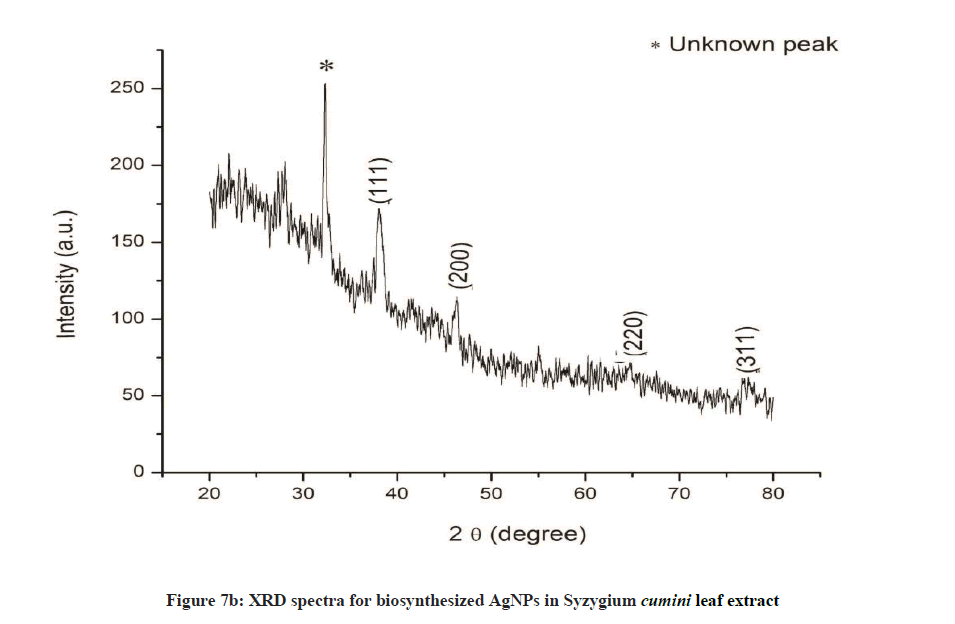
The crystalline structure of the synthesized nanoparticles was determined by XRD. The distinctive diffraction peaks obtained by XRD analysis are exhibited in Figures 6a and 6b. The four Bragg’s reflections planes were observed in the 2θ range of 30º-80º. These reflections were indexed as (111), (200), (220) and (311) atomic planes which are interpreted for the structure of metallic silver and they are in accordance to the Joint Committee on Powder Diffraction Standards (JCPDS, file no. 04-0783). This peak is due to crystallization of bio-organic compound present in plant leaves extract. The XRD analysis for A. bilimbi showed predominant diffraction peak of (111) along with two unknown peaks originated from the analyzed sample (Figure 6a). While S. cumini showed diffraction peaks of (111) along with one unknown peak (Figure 6b). These results were correlated with earlier reports.
FTIR- analysis
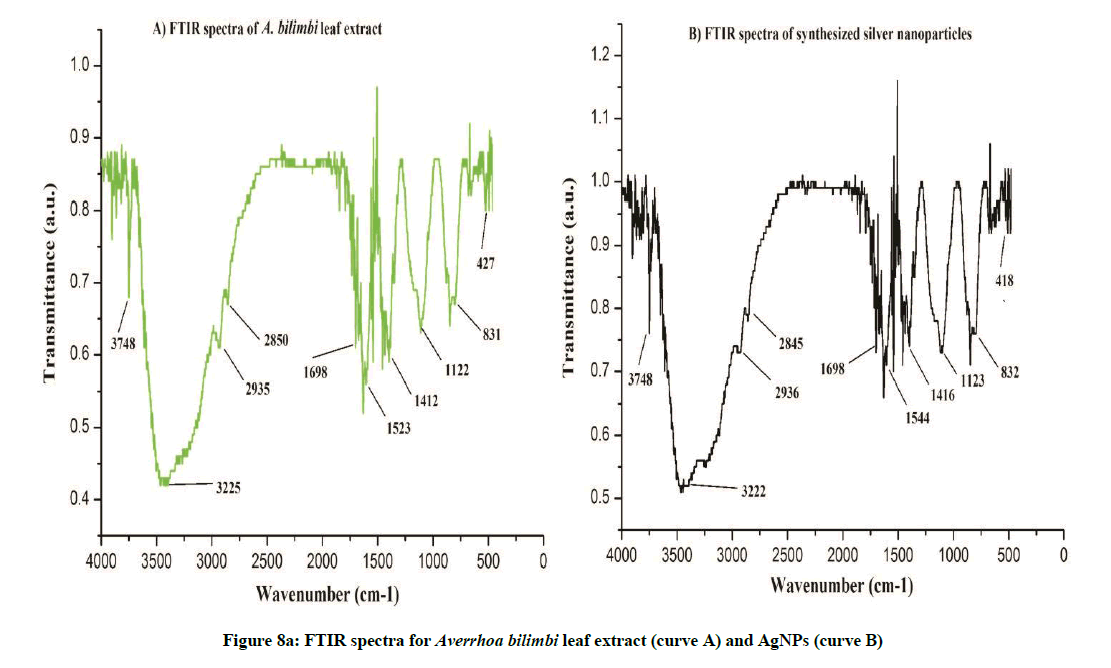
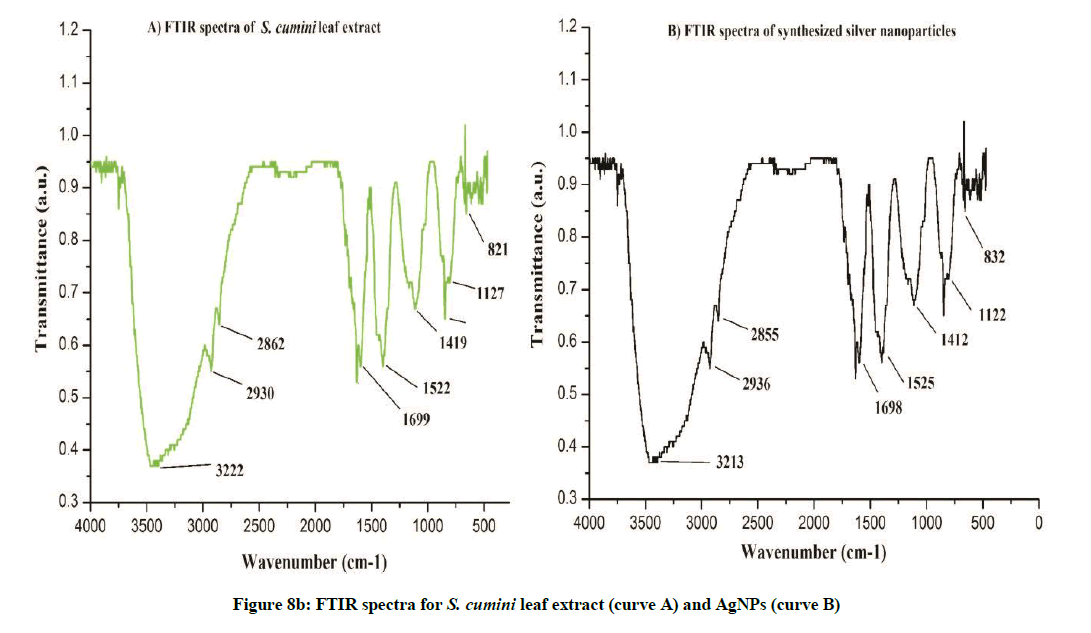
FTIR analysis was used to characterize the leaf extract and the synthesized nanoparticles (Figures 7a and 7b).The FTIR spectra for A. bilimbi and S. cumini leaf extract before and after bioreduction did not show significant changes in stretching frequencies of the FTIR spectrum. The FTIR spectrum for A. bilimbi leaf extract shows the band of several absorption peaks located at about, 418, 832, 1122, 1412, 1523, 1698 2850, 2935, 3225 and 3748 cm-1 in the region, 500-4000 cm-1. The prominent broad peak around 3748 to 3225 cm−1 is a characteristic of the hydroxyl functional group in alcohols and phenolic compounds. The FTIR spectra showed peaks at 2935 to 2850 cm-1 were assigned to C–H and C–H (methoxy compounds), alkanes groups stretching vibration. The peaks at 1698 to 1400 cm-1 range (1698, 1523, and 1412) are due to the presence of aromatic rings (aromatic C=C and conjugated carbonyl C=O) in the A. bilimbi leaf extract. The peaks in the range 1122 to 831 cm-1 are due to the presence of alky l halides, carbonyl and alcoholic groups in the components (Figure 8a). While S. cumini leaf extract showed several absorption peaks at 832, 1122, 1412, 1522, 1616, 1699, 2862, 2936, 3222 and 3750 cm-1 respectively (Figure 8b). The surface capping of their reported groups might be a curve for stability of synthesized nanoparticles in the studied biological green synthesis of SNPs. The existence of these functional groups proved the role of phytoconstituents in the synthesis, encapsulation and reduction of metal ion of synthesized AgNPs in both the plant extract. These findings of obtained spectrum for possible biological functional group in silver, gold and platinum nanoparticles were reported by Bar et al. [16]; Song et al. [17] and Kharat and Mendhulkar [1].
DPPH assay for antioxidant activity
The antioxidant activity of synthesized nanoparticles and plant leaf extracts was assayed by DPPH radical assay. This method is considered to be rapid, simple and most convenient. In the present assay, both nanoparticles and aqueous extracts of A. bilimbi and S. cumini exhibited rich scavenging effects on DPPH. The DPPH radical scavenging assay was evaluated using 10 mg/ml AgNP’s synthesized particles and 10 mg/mL leaf extracts (the aliquots were prepared as 50, 100, 150, 200, 250 μg/ml for AgNP’s and plant leaf extract), respectively. Ascorbic acid was used as a positive control (in the range of 20 to 140 μg/ml). A. bilimbi leaf extract with silver nanoscale particle indicated strong free radical scavenging activity with IC50 value of 150.47 μg/ml and scavenging ability in the range of 31.48 to 60.02% when compared with only leaf extract sample (IC50 value 214.33 μg/ml and scavenging ability range, 15.21% to 59.14%). In case of S. cumini, the leaf extract mediated AgNPs shows scavenging ability in the range of 22. 97 to 71.34% with average IC50 value, 152.07 μg/ml against leaf extract alone sample (scavenging ability 12.53% to 62.15% with IC50 value 188.76 μg/ml). Thus, for both the plants leaf extract mediated metal nanoscale particles exhibited more potential for free radical scavenging in over plant leaf extract. The free radical scavenging activity may be due to the presence of high phenolics, tannins or flavonoids constituent in the sample extracts (Table 1) [18].
| S. No. | Samples | Concentrations (in μg/ml) | Scavenging ability [in %] | IC50 value [in μg/ml] |
|---|---|---|---|---|
| 1 | Aqueous plant extract Averrhoa bilimbi | 50 | 15.21 ± 0.02 | 214.33 |
| 100 | 25.27 ± 0.06 | |||
| 150 | 34.04 ± 0.08 | |||
| 200 | 46.80 ± 0.08 | |||
| 250 | 59.14 ± 0.04 | |||
| 2 | Synthesized AgNP’s Averrhoa bilimbi | 50 | 31.48 ± 0.08 | 150.47 |
| 100 | 42.34 ± 0.03 | |||
| 150 | 52.19 ± 0.01 | |||
| 200 | 59.06 ± 0.06 | |||
| 250 | 65.02 ± 0.02 | |||
| 3 | Aqueous plant extract Syzygium cumini | 50 | 12.53 ± 0.09 | 188.76 |
| 100 | 23.29 ± 0.07 | |||
| 150 | 40.85 ± 0.06 | |||
| 200 | 59.57 ± 0.04 | |||
| 250 | 62.12 ± 0.06 | |||
| 4 | Synthesized AgNP’s Syzygium cumini | 50 | 22.97 ± 0.08 | 152.07 |
| 100 | 34.46 ± 0.06 | |||
| 150 | 55.55 ± 0.03 | |||
| 200 | 63.46 ± 0.09 | |||
| 250 | 71.34 ± 0.04 | |||
| 5 | Ascorbic acid (Standard) | 20-140 μg/ml | 81.35 ± 0.30 | 3.61 |
IC50: Concentration of the analyzed extracts causing 50% DPPH radical scavenging ability.
Table 1: Antioxidant activity of aqueous leaf extract mediated AgNPs, and ascorbic acid (standard) scavenging ability and IC50 value
Conclusion
Plant extract mediated silver nanoparticles were synthesized using two medicinally and economically important plants, A. bilimbi and S. cumini. These plants are well known for its fruits. The AgNPs were characterized and evaluated for their free radical scavenging potentials. Our findings indicated that the rate of synthesis of AgNPs is high in A. bilimbi compare to S. cumini. NTA results proved that A. bilimbi mediated SNPs has frequency of 61.30 particles/frame. While S. cumini shows the frequency of 77.22 particles/frame. The TEM analysis showed excellent conformity of nanoparticle shape, size and morphology. A. bilimbi SNPs revealed the variations in the particle size with the range of 10 nm to 50 nm with average of 50 nm. S. cumini revealed variations in the particles size from 7 to 20 nm with average of 20 nm. The XRD results showed crystalline nature of AgNPs. A. bilimbi showed reflection planes at (111), (200), (220) and (311) along with two unknown peaks. Similarly, S. cumini revealed reflection planes of (111), (200), (220) and (311) with one unknown peaks. FTIR analysis were carried out to identify the nature of possible biomolecules responsible for surface capping of the Ag nanoparticles synthesized in A. bilimbi and S. cumini leaf extract. The FTIR spectra for both the plants were compared and showed differences in the spectra due to the stretching of vibration of functional groups. The coating of functional groups like alcohols and phenols was observed for A. bilimbi mediated SNPs. The S. cumini leaf extract mediated Ag nanoparticles indicating the capping of alkyl halides, carbonyl and alcoholic groups. We believe that there phytoconstituents are playing key role to provide stability to the synthesized AgNPs. The stretching vibrations are due to these functional groups of bioactive compounds. The antioxidant activity of synthesized AgNPs and aqueous extract of in A. bilimbi and S. cumini leaf extract was comparatively studies along with ascorbic acid as a reference standard. Both the plant synthesized AgNPs showed superior antioxidant activity over respective leaf extract sample. Therefore, on the basis of results obtained, it can be concluded that both the plants have excellent potentials towards biological green synthesis of AgNPs which may be useful to produce effective antioxidants and formulations of nano-drug to cure variety of ailments. It will contribute to expose the further medicinal significance of these studied plant systems.
Acknowledgement
The authors are thankful to the Sophisticated Analytical Instrument Facility (SAIF), Indian Institute of Technology Powai, Mumbai. We also thank Dr. Rajesh Raut and Mr. Milind More for providing kind help for sample analysis.
References
- S.N. Kharat, V.D. Mendhulkar, Materials Sci. Engg. C, 2016, 62, 719-724.
- H.J. Prabu, I. Johnson, Karbala Int. J. Modern Sci., 2015, 1, 237-246.
- J. Fang, C. Zhang, R. Mu, Chem. Phys. Lett., 2005, 401, 271-275.
- Kathiravan, S. Ravi, S. Ashokkumar, Spectrochimica Acta Part A: Molecula. Biomol. Spectrosc., 2014, 130, 116-121.
- K.P. Bankura, D. Maity, M.M.R Mollick, D. Mondal, Bhowmick B, M.K. Bain, Carbohydrate Polymers., 2012, 89, 1159-1165.
- P.P. Gan, S.F.Y. Li, Environ. Sci. Biotechnol., 2012, 11, 169-206.
- N. Thamizhselvam, I.V. Liji, Y.R. Sanjayakumar, C.G. Sanal Gopi, K.G. Vasantha Kumar, Toxicol. Allied Clin. Pharmacol., 2015, 1, 2.
- Shu-Dong Wei, Hui Chen, Ting Yan, Yi-Ming Lin, Hai-Chao Zhou, LWT-Food Sci. Technol., 2014, 55, 278-285.
- G. Shui, L.P. Leong, Food Chem., 2006, 97, 277-284.
- S. Saha, E.V.S. Subrahmanyam, C. Kodangala, S.C. Shastry, Brazilian J. Pharmacog., 2013, 23, 651-661.
- J.M. Veigas, S.N. Mandayam, L.M. Padmere, B. Neelwarne, Food Chem., 2007, 105, 619-627.
- Y. Rout, S. Behera, A.K. Ojha, P.L. Nayak, J. Microbiol. Antimicrobials., 2012, 4, 103-109.
- V. S.B. Kashid, R.D. Tak, R.W. Raut, Colloids Surfaces B Biointerf., 2015, 133, 208-213.
- J. Huang, Q. Li, D. Sun, Y. Lu, Y. Su, X. Yang, H. Wang, Y. Wang, W. Shao, N. He, J. Hong, C. Chen, Nanotechnol., 2007,18, 105104.
- T. Santhoshkumar, A.A. Rahuman, G. Rajakumar, S. Marimuthu, A. Bagavan, C. Jayaseelan, Parasitol. Res., 2011, 108, 693-702.
- H. Bar, D.K. Bhui, G.P. Sahoo, P. Sarkar, S. Pyne, A. Misra, A: Physicochem. Eng. Aspects., 2009, 348, 212-216.
- J.Y. Song, E.Y. Kwon, B.S. Kim, Bioprocess Biosyst. Eng., 2010, 33, 159-164.
- Y.W. Mak, L.O. Chuah, R. Ahmad, R. Bhat, Journal of King Saud University Science., 2013, 25, 275-282.