Research - Der Pharma Chemica ( 2020) Volume 12, Issue 4
Spectrometric Estimation of Gliclazide, Nortriptyline, Cilnidipine in Pure and Tablet Dosage Form Using MBTH
Vijayalakshmi R*, Chamundeswari RN, Surya Kumari R and Suhasini SVijayalakshmi R, Department of Pharmaceutical Analysis, GIET School of Pharmacy, Rajahmundry-533296, Andhra Pradesh, India, Email: vijayalakshmigsp@gmail.com
Abstract
The objective of the present work is to develop simple, precise and accurate colorimetric methods for the estimation of Gliclazide (Method A), Nortriptyline (Method B), Cilnidipine (Method C) using MBTH (3-Methyl-2-Benzothiaazide Hydrazine) reagent. All the three Methods were developed on Perkin Elmer LAMBDA 25 UV/VIS spectrophotometer with 1cm quartz cells. Quantification of Gliclazide, Nortriptyline, Cilnidipine were done by Oxidative coupling method using MBTH as reagent. Gliclazide (Method A), Nortriptyline (Method B) and Cilnidipine (Method C) undergoes oxidation with ferric chloride and couples with MBTH. The coupled oxidant shows maximum absorbance measured at 660 nm for Gliclazide (Method A), 650 nm for Nortriptyline (Method B), and 600 nm for Cilnidipine (Method C). The colorimetric method was extensively validated as per ICH guidelines and all the parameters were within the acceptance criteria with correlation of 0.999% RSD less than 2 for the method. The method was proved to be more accurate, simple, precise and rapid by statistical validation as well as recovery studies and can be used for routine laboratory analysis.
Keywords
Gliclazide, Visible spectrophotometry, Validation, Gliclazide, Nortriptyline, Cilnidipine, MBTH.
Introduction
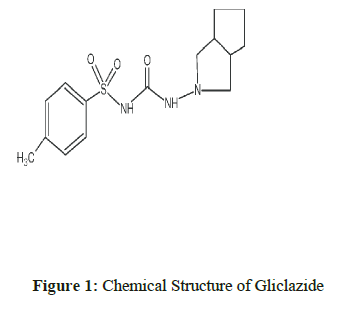
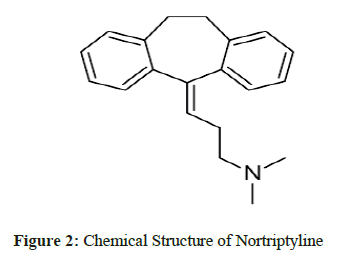
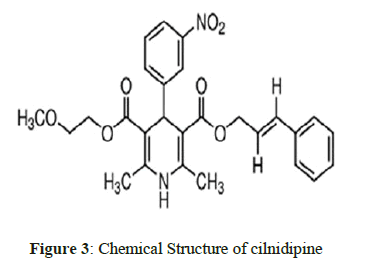
Gliclazide1, chemically is 1-(3-azabicyclo (3.3.0) oct-3-yl)-3-(p-tolylsulfonyl) urea is an oral antihyperglycemic agent used for the treatment of non-insulin- dependent diabetes mellitus (NIDDM). Gliclazide binds to the β cell sulfonyl urea receptor (SUR1). Nortriptyline2, chemically is 3-(10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5-ylidene)-N-methyl-1-propanamine. It is the active metabolite of amitriptyline, is a tricyclic antidepressant (TCA). It is used in the treatment of major depression and is also used off-label for chronic pain and other conditions. Cilnidipine3, chemically is 1,4-dihydropyridine-3,5-dicarboxylic acid. It is the L-type calcium channel blocker [1-3].
The Chemical structures of Gliclazide, Nortriptyline and Cilnidipine are shown in Figure 1, 2 & 3 respectively. Literature survey of these drugs revealed that there are few methods for the determination of Gliclazide/Nortriptyline/Cilnidipine alone by HPLC and spectrophotometric methods [4-19]. The existing HPLC methods take more time for sample preparation & analysis, at the same time expensive. The reported UV methods may not be specific as there is no procedure for elimination of interference by the additives or active pharmaceutical ingredients. Gliclazide and Nortriptyline were chosen for the presence of active methylene groups. As such Cilnidipine would not react with MBTH to give colored product but the presence of nitro group could be helpful to reduce to amino group which helps for analysis by oxidative coupling with MBTH. There were no reported methods for the proposed drugs by visible spectrophotometry using ferric chloride and MBTH. The purpose of this work is to develop visible methods focused on economic point of view as well as stable and short analysis time by green solvents for routine analysis and to validate the methods according with ICH guidelines.
Materials and Methods
Equipment
Double-beam Perkin Elmer (LAMBDA 25) UV-Vis spectrophotometer was used for spectral measurements. Sarotius electronic balance was used for weighing the chemicals.
Chemicals
Gliclazide, Nortriptyline, Cilnidipine are obtained as a gift sample from Aurobindo pharma Ltd, Hyderabad. Triple distilled water, MBTH (3-Methyl-2-Benzothiaazide Hydrazine), sodium hydroxide, methanol and ferric chloride were used for the experimental work. All the chemicals used in the experimental procedure are of analytical reagent grade.
Preparation of Solutions
Preparation of stock solution for estimation of Gliclazide (Method A)
About 25 mg of Gliclazide was weighed and transferred to a 25 ml volumetric flask, 0.1 N NaOH was added to dissolve it and diluted to volume with 0.1 N NaOH. The resulting solution has a concentration of 1 mg/ml.
Preparation of stock solution for estimation of Nortriptyline (Method B)
About 25 mg of Nortriptyline was weighed and transferred to a 25 ml volumetric flask, Ethanol was added to dissolve it and diluted to volume with Ethanol. The resulting solution has a concentration of 1 mg/ml.
Preparation of stock solution for estimation of Cilnidipine (Method C)
About 10 mg of Cilnidipine was weighed and transferred to a 10 ml volumetric flask, then 5 mg of zinc and 0.2 ml of 5N HCl was added for reduction. When the effervescence ceases the solution was filtered, final volume was made with methanol. The resulting solution has a concentration of 1 mg/ml of reduced Cilnidipine.
Preparation of 0.5% MBTH (3-Methyl-2-Benzothiaazide Hydrazine)
About 50 mg of MBTH (3-Methyl-2-Benzothiaazide Hydrazine) was dissolved in distilled water and made to 100 ml with the same solvent.
Preparation of 0.5% Ferric chloride (FeCl3)
About 50 mg of Ferric chloride (FeCl3) was dissolved in distilled water and made to 100 ml with the same solvent.
Procedure for Calibration Plot
Method A
Into a series of 10 ml volumetric flasks, 0.4 ml-1.4 ml of working standard solution of Gliclazide was pipetted out and 0.6 ml of MBTH and 1 ml of FeCl3 were added, then the final volume was made to 10 ml with distilled water. The absorbance of the greenish colored chromogen was measured at 660 nm against the reagent blank. The amount of Gliclazide present in the sample solution was computed from its calibration curve.
Method B
In a series of 10 ml volumetric flasks, 0.2 ml-1.0 ml of working standard solution of Nortriptyline was pipetted out and 0.8 ml of MBTH and 1 ml of FeCl3 were added, then the final volume was made to 10 ml with distilled water. The absorbance of the bluish colored chromogen was measured at 650 nm against the reagent blank. The amount of Nortriptyline present in the sample solution was computed from its calibration curve.
Method C
In a series of 10 ml volumetric flasks, 0.4 ml-1.2 ml of working standard solution of Cilnidipine was pipetted out, then add 1 ml of MBTH and 1ml of Fecl3 were added, then the final volume was made to 10 ml with distilled water. The absorbance of the greenish colored chromogen was measured at 600 nm against the reagent blank. The amount of Cilnidipine present in the sample solution was computed from its calibration curve.
Assay Procedure
Method A
Twenty tablets of commercial samples (Reclide 80mg TAB) of Gliclazide were accurately weighed and powdered. Tablet powder equivalent to 10 mg of Gliclazide was dissolved in 10 ml 0.1 N NaOH, filtered. The resulting solution has a concentration of 1 mg/ml then the procedure given for pure drug analysis was followed.
Method B
Twenty tablets of commercial samples (Nortri-Tab 25 mg) of Nortriptyline were accurately weighed and powdered. Tablet powder equivalent to 25 mg of Nortriptyline was weighed and transferred to a 25 ml volumetric flask, Ethanol was added to dissolve it and diluted to volume with Ethanol. The resulting solution has a concentration of 1 mg/ml.
Method C
Twenty tablets of commercial samples (Ciledge Tab 10 mg) of Cilnidipine were accurately weighed and powdered. Tablet powder equivalent to 10 mg of Cilnidipine was weighed and transferred to a 10 ml volumetric flask, then 5 mg of zinc and 0.2 ml of 5N HCl was added for reduction. When the effervescence ceases the solution was filtered, final volume was made with methanol. The resulting solution has a concentration of 1 mg/ml of reduced Cilnidipine.
Discussion
The proposed visible spectrophotometric techniques are considered to be simple, less expensive and reliable by which it predominates the application of sophisticated techniques for regular analysis. Among the proposed methods, method B was more sensitive but range of linearity is medium. Methods A & C shows better linearity range between 40 μg/ml-250 μg/ml. Also, by the LOD and LOQ data from Table 3 it proves that method B is more sensitive due to very low values compared to other methods A & C. The sensitivity and selectivity of the colorimetric methods depend on the nature of the chemical reactions involved in color development and not on the sophistication of the equipment. The novelty of the method lies in the selection of appropriate chromogenic reagents for their assay by exploiting their characteristics, (physical and chemical properties) based on the oxidizable functional groups present in drug molecule. The choice of the chromogenic reagent for color development on a particular method is still a challenging problem. Selection of the chromogenic reagent is based on the presence of active methylene group in Gliclazide and Nortriptyline. Even though there is a heterocyclic amine in Cilinidipine, but it could not produce the oxidative coupled product with MBTH. But after the reduction of nitro group to amino group, Cilnidipine showed very good response with MBTH. This proves the specificity of the method. The careful consideration of factors such as the presence of other functional groups besides the chosen one that might be adversely affected by the reagents for the specificity study, the instability of desired colored product for stability of colored product, the rate of reaction and other related factors were all studied and the evidences presented in results section proved that the methods are specific, sensitive, linear. The validation studies proved the methods to be rugged, robust and accurate.
Conclusion
Even though there are some methods for the determination of Gliclazide, Nortriptyline, Cilnidipine by HPLC and spectrophotometric methods, the proposed colorimetric methods are simple, economic and sensitive with reasonable precision, accuracy and constitute better alternative to the existing ones for the routine determination in bulk and pharmaceutical formulations.
Acknowledgements
The authors are thankful to the management for providing the laboratory facilities to conduct the research work.
Author Contributions
Concept – V.R; Design – V.R; Supervision – V.R; Resources – GIET School of Pharmacy, Materials – RN.C., S. R, S. S; Data Collection and/or Processing – RN.C., S. R, S. S.; Analysis and/or Interpretation – RN.C., S. R, S. S.; Literature Search – RN.C., S. R, S. S; Writing – V.R, RN.C., S. R, S. S.; Critical Reviews – V.R, RN.C., S. R, S. S.
Conflict of Interest Statement
The authors declared no conflict of interest.
References
[1] The British Pharmacopoeia, HSMO, London, 2004, Vol. I, III, IV,
[2] www.Drugbank.com/Nortriptyline Hydrochloride
[3] O’Neil MJ, The Merk Index, An Encyclopedia of Chemicals, Drugs and Biologicals, 14th ed. Merck & Co. Inc.; 2006:2275,6839
[4] V. Darshan, P. Daxesh Patel, V. Jaivik Shah et al., Determination of gliclazide in human plasma by UPLC-MS/MS and its applications to a bioequivalence study. J Modern Drug Dis and Drug Delivery Res. 2014, 2: p. 1-9.
[5] BVV Ravi kumar, AK Patnaik, Saroj Kumar Raul et al., RP-HPLC ethod development and validation for the estimation of gliclazide in bulk and pharmaceutical dosage forms. J App Pharm Sci, 2013, 3: p. 059-062.
[6] KP Dadhania, AN Parthika, Yadvendra KA. Development and validation of spectrophotometric method for simultaneous estimation of gliclazide and metformin hydrochloride in bulk and tablet dosage form by simultaneous equation method. Int J Pharm Sci Res, 2011, 2: p. 1559-1563.
[7] R. Talari, J. Varshosaz, A. Mostafavi et al., Development and validation of a novel RP-HPLC method for pharmacokinetic studies of Gliclazide in rat. Farmacia, 2011, 59: p. 388-395.
[8] PN Dhable and CR Seervi. Simultaneous UV Spectrophotometric method for estimation of gliclazide and metformin hydrochloride in tablet dosage form. Int j ChemTech Res. 2010, 2: p. 813-817.
[9] R. Bhanu, SK Kulkarni and AB Kadam. Simultaneous estimation of Gliclazide and Metformin in pharmaceutical dosage by reverse phase high performance liquid chromatography. Indian Drugs. 2006, 43: p. 16-20.
[10] S. Gayatri, A. Shantha, V. Vaidhyalingam et al., Simultaneous estimation of Gliclazide and Rosiglitazone from its pharmaceutical dosage form by HPLC method. Indian Drugs. 2004, 41: p. 374-375.
[11] MR Rouini, A. Mohajer and MH Tahami. A simple and sensitive HPLC method for determination of gliclazide in human serum. J Chromatogr B. 2003, 785: p. 383-386.
[12] SI. Sa'sa and I. Jalal. Determination of nortriptyline hydrochloride and fluphenazine hydrochloride in commercial tablets by reverse phase high-performance liquid chromatography. Microchem. J., 1988, 38: p. 181-187.
[13] NA El-Ragehy, SS. Abbas and SZ. El-Khateeb. Spectrophotometric and stability indicating high performance liquid chromatographic determination of nortriptyline hydrochloride and fluphenazine hydrochloride Anal. Lett. 2002, 35: p. 1171-1191.
[14] NA El-Ragehy, SS Abbas and SZ El-Khateeb. Stability indicating method for determination of nortriptyline hydrochloride using 3-methyl-2-benzothiazolinone hydrazone (MBTH). J. Chromatogr. A, 2001, 923: p. 107-117.
[15] H. Mahgoub, MA. Korany, H. Abdine et al., Spectrophotometric assay of fluphenazine HCl–nortriptyline HCl mixture in tablets using fourier function method Anal. Lett. 1991, 24: 1797-1811.
[16] KS. Kokilambigai and KS. Lakshmi. Analytical methodologies for determination of Cilnidipine: An Overview. Int. J Pharm Pharm Sci. 2014, 6: 36-38
[17] P. Ashish, P. Arti, P. Viral et al., FTIR spectroscopic method for quantitative analysis of cilnidipine in tablet dosage form. Int. J Pharm Sci Res. 2015, 6:1033-39.
[18] P. Pankaj, A. Chaudhari, V. Bhalerao. Method validation for spectrophotometric estimation of cilnidipine. Int J Pharm Sci. 2013, 4: p. 96-98.
[19] M. Mohammed Safhi. Spectrophotometric estimation of Cilnidipine in tablets. British J Pharm Res. 2015, 7: p. 451-456.