Research Article - Der Pharma Chemica ( 2020) Volume 12, Issue 7
Spectrophotometric determination of Darunavir using NQS and Brucine meta periodate
Acharyulu MLN1*, Mohana Rao PVSR2 and Siva Rama Koti I32Department of Engineering Chemistry, A. U. College of Engineering(A), Visakhapatnam-530003, AP., India
3Department of Chemistry, Ceturion University of Technology and Management, Parlakhimundi, Odisha - 761211, India
Acharyulu MLN, Department of Basic Sciences and Humanities, Ceturion University of Technology and Management, Andhra Pradesh, India, Email: acharyulu@cutmap.ac.in
Received: 31-Jul-2020 Accepted Date: Nov 22, 2020 ; Published: 28-Nov-2020
Abstract
Two simple and sensitive spectrophotometric methods (A and B) for the assay of Darunavir (DNV), in pure and pharmaceutical formulations were developed. The method-A is based on the condensation reaction of DNV forming an orange-red colored Schiff's base of maximum absorption peak (λ max) at 444 nm with sodium 1,2-naphthaquinone-4-sulfonate (NQS) in an alkaline medium. The dimethoxy benzene nucleus of brucine is attacked by IO4- with the formation of O-quinone (Bruci quinone) which in turn undergoes nucleophilic attack on the most electron rich position of the coupler i.e., proton bearing amino group (primary amine of DNV), to give 1-mono substituted Bruci quinone derivative. The reaction mechanism in both methods were discussed. The absorbance of colored complex is found at 511nm. Beer's law is obeyed in the concentration range 10-60 μg/ml, 50-300 μg/ml, the Molar absorptivity values are1.3874x 105, 2.9865x 104 L/mol.cm, Correlation Coefficients are 0.9988,0.9996, Sandell sensitivity are 3.9473x 10-3, 1.8337 x 10-2ng/cm2 .The methods proposed gave reproducible results with the percentage recoveries in the formulations found to be 99.812 to 99.749 and 99.682 to 99.774 for Methods A and B.
Keywords
Spectrophotometry; NQS; Bruci quinone, Per Iodate, Pharmaceutical formulations.
Introduction
Darunavir (DNV), (Figure 1), is an oral anti-retroviral agent which selectively inhibits the cleavage of Human immunodeficiency virus (HIV-1) encoded Gas-polyproteins in infected cell, thereby preventing the formation of mature virus. Darunavir ethanolate is chemically [(1S,2R)-3-[[(4-amino phenol) sulfonyl](2-methyl propyl)amino]-2-hydroxy-1- (phenylmethyl)propyl]-carbamic acid (3R,3aS,6aR)-hexahydro furo[2,3-b]furan-3-yl ester mono ethanoate [1].
This drug is effective in patients experienced in anti-retroviral treatment, such as those carrying HIV-1 strains which are resistance to more than API [2]. Literature survey revealed that different analytical methods have been reported for the determination of DNV in plasma using liquid chromatography coupled with tandem mass spectroscopy [3-4] simultaneous determination of DNV with other anti-retroviral agents in plasma [5,6]. Few HPTLC methods for determination of DNV in rat plasma and in tablet dosage form its application to pharmacokinetics studies [7]. Infrared Spectroscopy method for determination of DNV in tablets [8]. Few methods have been developed for determination of DNV by HPLC [9-13]and Electrophoretic method for the separation of DNV [14]and Spectrophotometric methods [15-22]. The analytical useful functional groups in DNV have not been fully exploited for designing suitable visible spectrophotometric methods and so still offer a scope to develop more visible spectrophotometric methods with better sensitivity, precision and accuracy. The author has made some attempts in this direction and succeeded in developing proposed methods. All these methods have extended pharmaceutical formulations as well. Literature survey reveals that reported HPLC methods require more time for sample analysis resulting in lesser throughout. Krishna Kumar Rao et al have estimated Darunavir Ethanolate by spectrophotometry [23]. The author investigated the role of various reagents used [26-29]. Upon thorough literature survey done by the author,it is clear that no attempt has been made by earlier authors to make use of useful functional groups in DNV for its determination by visible spectrophotometric methods
Instruments Used
A Schimadzu UV-Visible spectrophotometer 1801 with 1 cm matched quartz cells was used for all spectral and absorbance measurements. A Systronics digital pH meter 361 was used for pH measurements.
Preparation of standard Drug solution
The stock solution (1 mg/ml) of DNV was prepared by dissolving 100 mg of it in 100 ml of milli pore distilled water. A portion of this stock solution was diluted stepwise with the distilled water to obtain the working standard DNV solution of concentrations 4-600 μg/ml.
Procedure of Assay of DNV in formulations
An accurately weighed amount of formulation (tablet) equivalent to 100 mg of drug was dissolved in 20 ml of distilled water, shaken well and filtered. The filtrate was further diluted to 100 ml with distilled water to get 1 mg/ml solution of drug in formulations.
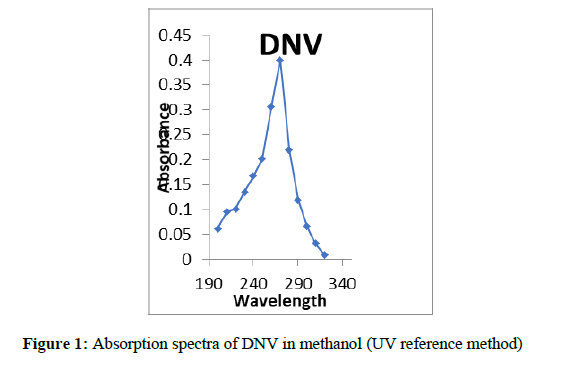
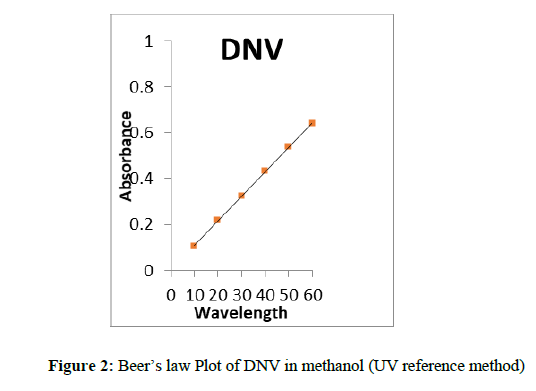
One ml of this solution was furthered diluted to 25 ml to get 40 μg/ml solution. The absorbance of the solution was determined λ max 223 nm (Figure 1). The quantity of the drug was computed from the Beer’s law plot (Figure 2) of the standard drug in distilled water.
Recommended Procedures
After systematic and detailed study of the various parameters involved, as described under results and discussion in this chapter, the following procedures were recommended for the determination of DNV in bulk samples.
Method-A
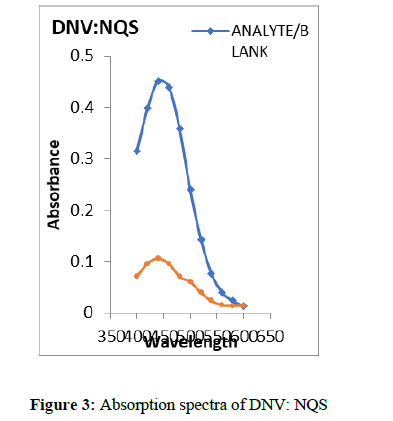
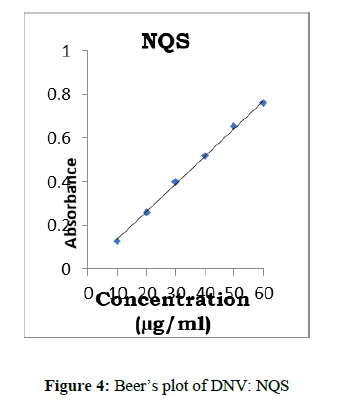
Aliquots of standard DNV solution (0.1-0.6 ml, 60 μg/ml) were placed into a series of 20 ml test tubes. Then 1.0 ml of NQS and 1.0 ml of NaOH solutions were added to each tube and kept aside for 2 min. at lab temperature. The solutions were made up to the mark with distilled water. The absorbances were measured at λmax 444 nm (Figure 3) against a reagent blank prepared simultaneously. The amount of DNV in a sample was obtained from the Beer-Lambert’s plot (Figure 4).
Method-B
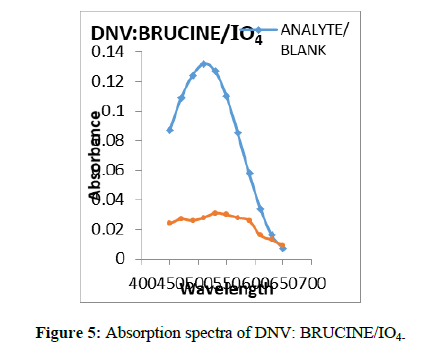
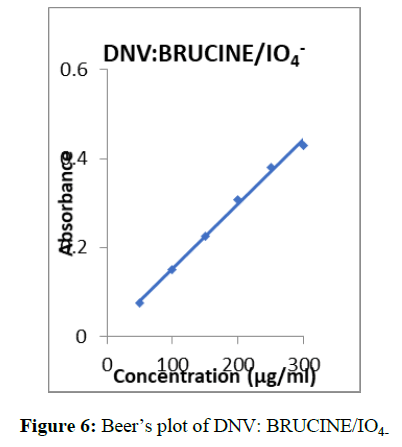
Into a series of 20 ml calibrated tubes, aliquots of standard DNV solution (0.1 – 0.6 ml, 300 μg/ml) were taken. Then 3.0 ml of brucine solution and 1.5 ml of IO4- solution were added successively. The flasks were kept aside for 2 min. and then 2.0 ml of Sulphuric acid was added and then heated the contents on boiling water bath for 15 min. The flasks were cooled to room temperature and made up to the mark with distilled water. The absorbance of the coloured species was measured after 5 min at λ max 519 nm (Figure 5) against the reagent blank. The amount of DNV was computed from its calibration graph (Figure 6).
Chemistry of coloured species in the present investigation
DNV possesses different functional moieties such as primary amine, tertiary amine and sulphonyl groups of varied reactivity. Tertiary amine undergoes nucleophilic attack of dimethoxy benzene nucleus forms oxidative coupling with Brucine/IO4-
Method-A
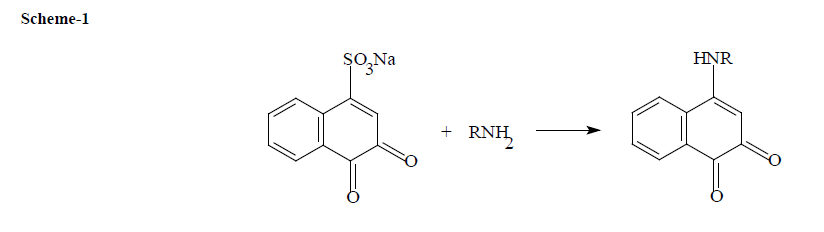
In this method, the presence of primary amino group in DNV permits the development of new spectrophotometric method for its determination through the formation of coloured nucleophilic substitution reaction product with NQS. The reactions of DNV with NQS are described in the scheme-1
Method-B
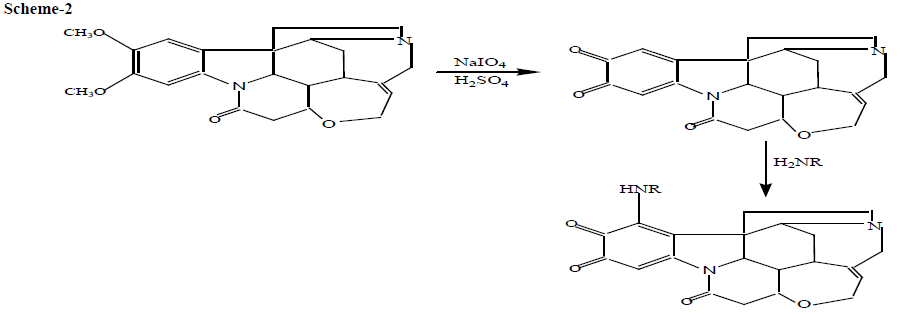
The dimethoxy benzene nucleus of brucine is attacked by IO4- with the formation of O-quinone (Bruci quinone) which in turn undergoes nucleophilic attack on the most electron rich position of the coupler i.e., proton bearing amino group (primary amine), to give 1-mono substituted Bruci quinone derivative. The reactions of DNV with brucine in the presence of IO4- are described in the scheme -2.
Conclusion
The results presented above indicate that the proposed methods have good sensitivity, selectivity, precision and accuracy. Results of analysis of bulk form and formulations reveal that the proposed methods are suitable for the estimation of DNV in them, as impurities and excipients present in them cause no interference virtually.
References
[1] Drug bank available from http: www.drugbank.Ca drug. /db01264[last accessed on 2015.
[2] N Clotet, JM Bellos, D Molina et al. 2007, 369: p. 1169-1178,
[3] W Galen, wing. Mc Graw Hill International Edition, 48: p. 378.
[4] Amit Patel, Ami Patel and Ashlesha Makwana. Int. J. Res Pharm. Bio. sci. 2013, 4: p. 1138-1147
[5] Nageshwar Rao, Ram chandra, Santosh kumar. J Pharm Biomed Anal. 2013,75,186-191
[6] M Avolio, M. Siccardi, L Sciandra et al. J Chromatogr B. 2007, 859: p. 234-240,
[7] K Hari Babu, Sisla Ramakrishna, Kiran kumar. J. Liq. Chromatography Releated Technol. 2013, 36: p.169-179
[8] Ana Carolina Kongana, Herida Regina. Phy. Chem, 2013, 3: p.1-6
[9] G Raveendra Babu, A Lakshmana Rao and J Venkateshwara Rao. Int. J. Res. Pharm. Chem. 2013, 3: p. 438- 443
[10] N Bhavani, B Patel, N Suhagia. International Journal of Pharm Tech Reseach. 2012, 4,450-456
[11] B Raveendra, G amprasad, A Lanka. Asian J. Pharm. Res. 2011, 1, p. 10-14
[12] L Satyanarayana, SV Naidu, M Narasimha Rao. Asian J. Res. Pharm. Sci. 2011,1: p. 74-76
[13] B Manisha, J Pranali, V Anuja et al., Int.J.Pharm.Sci.,2013, 21, p. 20-23
[14] B Gholve Sachin, S Asware Baburao, C dam Shrihari et al. World J. Pharm. Res. 2015, 4: p. 1276-1283
[15] S Leonard, A Schepdael, T Lvanyi et al., Electrophoresis. 2005, 26: p. 627
[16] NA Ragehy, SS Abbas and SZ Khateeb. J. Pharm. Biom. Anal. 2001,25: p. 143-151
[17] Suryaprakasa Sastry, A Rama Mohana Rao and TNV Prasad. J. Analutical Letters. 1987, p. .20
[18] K Sowjanya, JC Thejaswini J. Gurupadayya. Der Pharma Chemica. 2011, 3: p. 112-122
[19] RS Chandan, M Vasudevan, BM Deecaraman et al. J. Pharm. Res. 2011,4: p. 1813-1815
[20] JN Rodriguezlopez, J Escribano and F Garciacanovas. Analytical Biochemistry. 1994, 216: p. 205-212,
[21] DA Pani Kumar, G Archana, G Sunitha, Pharmaceutica Analytica Acta. 2015, 6: p. 1-5
[22] Shah, B Paresh, K Pundarikakshudu, 2006,89: p. 987-994,
[23] KVV Krishna Kumar Rao, B Phanindra, K Rajesh. Inventi Rapid Pharma Analysis and &quality Assurance. 2013,4.
[24] International Conference on Harmonization (ICH), Q2 (R1), November 2005.
[25] Authority of the United States Pharmacopeial Convention. The United States Pharmacopeia (USP34), National Formulary (NF 29). Maryland., 2011.
[26] R.Vijayalakshmi, Y. Naga Sri Ramya, A. Dimple Mani et al., Int. J. Pharm Tech Res. 2016,6: p.301-306
[27] T Manikya Sastry, U Sujana Kumari, KV Nagalakshmi. Int. J. Adv. Res. Sci. Eng. 2017,6(8).
[28] M. Purushotham Reddy, N. Rami Reddy. Int. J. Chem. Sci. 2013,11(1): p. 614-618
[29] PVSR. Mohana Rao, K Raghu Babu, VR. Murthy et al. Der Pharmacia Letter. 2016, 8(5): p. 354-361