Research Article - Der Pharma Chemica ( 2017) Volume 9, Issue 3
Stability Indicating Method Development and Validation of Lamivudine, Zidovudine and Nevirapine by Using HPLC
Som Shankar Dubey* and Mahesh DuggiralaSom Shankar Dubey, Department of Chemistry, Gitam Institute of Technology, Gitam University, Visakhapatnam, India,
Abstract
A simple, efficient, and robust stability-indicating RP-HPLC method has been developed and validated to measure Lamivudine, Zidovudine and Nevirapine at a single wavelength (268 nm) in order to assay. The samples were eluted in an isocratic method using an inertsil ODS column (4.6 mm × 250 mm with a particle size of 5 μ) with a mobile phase consisting of 10 mM Ammonium acetate buffer (with a pH adjusted to 3.8 using Acetic acid): acetonitrile (60:40, v/v), acetonitrile and water (1:1, v/v) using as diluent, through ambient temperature delivered at a flow rate 1.2 mL/mins. A good linear response was obtained in the range from 15-75 μg/mL, 30-150 μg/mL and 20-100 μg/mL of Lamivudine, Zidovudine and Nevirapine respectively. The LODs for Lamivudine, Zidovudine and Nevirapine were found to be 0.315, 0.405 and 0.600 μg/mL, respectively and the LOQs for Lamivudine, Zidovudine and Nevirapine were 0.945, 1.080 and 2.100 μg/mL respectively. The method was quantitatively evaluated in terms of accuracy (recovery), linearity, precision, selectivity and robustness in accordance with standard guidelines. The method is simple, suitable and conducive for analyzing Lamivudine, Nevirapine and Zidovudine in bulk and in pharmaceutical formulations.
Keywords
Degradation, HPLC, Lamivudine, Zidovudine, Method development, Validation, Nevirapine
Abbreviations
NRTIs: Nucleotide Reverse Transcriptase Inhibitors; NNRTI: Non-Nucleotide Reverse Transcriptase Inhibitors; UV Detector: Ultraviolet Detector; RSD: Relative Standard Deviation; HPLC: High-Performance Liquid Chromatography; ICH: International Conference on Harmonization; SD: Standard Deviation; PDA: Photodiode array; LOD: Limit of Detection; LOQ: Limit of Qualification; DNA: Deoxyribonucleic acid; RNA: Ribonucleic Acid; HIV: Human Immunodeficiency Virus; LC: Liquid Chromatography; LC-MS: Liquid Chromatography-Mass Spectroscopy; USP: United States Pharmacopeia; AIDS: Acquired Immunodeficiency Syndrome; Rt: Retention time; RT: Room Temperature
Introduction
A disease caused by human immunodeficiency virus (HIV) [1-3] is called Acquired Immune Deficiency Syndrome (AIDS) which affects the human immune System. It is the critical clinical effect of infection with HIV, which is a retrovirus that directly and indirectly destroys CD4+ T cells [4]. Present treatment for HIV infection contains highly active antiretroviral therapy that reduces mortality and morbidity of HIV infection Patients [5,6]. The remedy for the treatment of HIV infection is triple drug therapy with a two Nucleoside Analogue Reverse Transcriptase Inhibitors (NRTIs) backbone in combination with a protease inhibitor or a Non-Nucleoside Reverse Transcriptase Inhibitor (NNRT) [7,8]. Both NRTI and NNRTI here inhibit an activity of reverse transcriptase, which is an essential viral enzyme that transcribes viral RNA into DNA. In developing countries, patients are using fixed-dose tablets containing the combination of lamivudine and nevirapine with either Stavudine or Zidovudine [9]. Both Lamivudine and Zidovudine are NRTIs. Zidovudine significantly slows HIV spread, but doesn’t stop entirely [10]. This allows HIV to become Zidovudine-resistant over time, for this reason, Zidovudine is usually used in conjunction with other NRTIs and anti-viral drugs to prolong the lifespan of AIDS patients [11-13]. It is highly synergistic to use Lamivudine in combination with Zidovudine. Nevirapine belongs to the class of NNRTI and it is recommended for antiretroviral therapy. It is more effective to use in a combination of three or more antiretroviral drugs as HIV quickly develops resistance to the single antiretroviral drug if it used alone.
In the literature, numerous methods are described to determine separately or in combination of lamivudine, zidovudine and nevirapine with other drugs in pharmaceutical formulation [14-22] still, very few methods are reported to determine nevirapine, zidovudine and lamivudine simultaneously in biological matrices by using High-Performance Liquid Chromatography (HPLC) or LC-MS/MS [23-32].
Our aim was to develop a simple, accurate, sensitive method for simultaneous determination of nevirapine, zidovudine and lamivudine in combined pharmaceutical dosage form by HPLC with UV detection, where simple mobile phase composition was used for chromatographic separation without any ion-pairing agent. Total retention time for analysis was short with a good resolution between these components. All these reasons make this new method was really lucrative. This method was also validated for linearity, accuracy, precision, selectivity, sensitivity and degradation studies according to the ICH guidelines.
Experimental
Chemicals and reagents
Lamivudine, Zidovudine and Nevirapine were obtained from Pharmatrain (Kukatpally, Hyderabad), chemical structures were shown in Figures 1-3. Ammonium acetate, Acetic acid and acetonitrile were used HPLC Grade obtained from Merck (Mumbai, India). Milli-Q-Water resistivity 18.2 MΩ × cm was generated from a Milli-Q-Water purification system manufactured by Millipore (USA).
Equipment
The HPLC instrument (waters alliance 2695 model) consisted of Quaternary pump (model 2790), low pressure mixing pump and inline vacuum degassing, Flow rates of this system from 50 μl/mins to 5 ml/mins, the maximum capacity of Autosampler has 120 vials with temperature control from 4ºC to 40ºC, column compartment provides ambient to 65ºC and Photodiode array detector (PDA) (model 2996) with a wavelength range of 190-800 nm. The output signal was recorded on the monitor and then quantified by using Empower 2 Software.
Chromatographic conditions
The Chromatographic analysis was performed with an isocratic elution mode for 10 mins run time, with ambient column temperature. The mobile phase consists of 10 mM Ammonium acetate and adjusted pH: 3.8 with Acetic acid- acetonitrile (60:40), the flow rate of pump set 1.2 mL, Inertsil ODS column (length 250 mm × 4.6 inner diameter, 5 μ particle Size), the chromatogram was monitored with UV detection at 268 nm and injection volume 4 μl. Water and acetonitrile in the ratio of 50:50 used as diluents.
Methodology
Preparation of standard solution
Stock Standard Solution was prepared by taking accurately weighted 15 mg, 30 mg and 20 mg of lamivudine, Zidovudine and Nevirapine working standards into 10 mL cleaned and dried volumetric flask, add diluent let it be dissolved completely and using the same diluent make volume up to the mark. Taken 1.0 mL of the above solution into 10 mL volumetric flask and add diluent to make it up to the mark. For preparing the standard Solution, taken 3.0 mL of solution from above stock solution into 10 mL volumetric flask and add diluent to make it up to the mark.
Assay of pharmaceutical dosage form (sample solution)
For the preparation of the sample solution, the samples of Lamivudine, Zidovudine and Nevirapine are taken in the concentration of 0.045 mg/mL, 0.09 mg/mL and 0.06 mg/mL respectively. The process of preparation is the same as standard solution.
Assay of pharmaceutical formulation
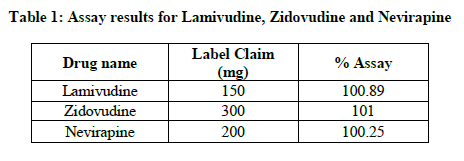
The proposed method was effectively applied to find Lamivudine, Zidovudine and Nevirapine in their tablet dosage form. The results obtained for Lamivudine, Zidovudine and Nevirapine were comparable with the consequently labeled amounts, results were shown in Table 1.
Method validation
According to the International Conference on Harmonization (ICH) guidelines, this method was validated.
Linearity
Calibration plots for the analytes were prepared with standard stock solutions to yield the concentration of 15-75 μg/mL for Lamivudine, 30-150 μg/mL for Zidovudine, 20-100 μg/mL for Nevirapine into the HPLC system. Taken five concentrations in between the ranges given above, and performed triplicate injections of each concentration. Calibration curves were plotted between concentrations of analytes versus area of that analyte. Linearity regression analysis of the data gave slope, intercept and correlation coefficient value.
Accuracy/Recovery
Standard addition technique was used to perform accuracy by recovery studies. The pre-analyzed samples were spiked with extra 50%, 100%, 150% of each standard Lamivudine, Zidovudine, Nevirapine and the mixtures were analyzed by the method proposed. The recovery studies were conducted in triplicate.
Precision
For the precision, repeatability was carried out for a short time interval under the same chromatographic parameters. For the intermediate precision, repeatability was carried out in a different day under same chromatographic parameters. The sample with the same concentration was injected in six replicates for intraday (precision) and six replicates interday (intermediate precision). The peak area for injection recorded, and calculated RSD for intraday (precision) and interday (intermediate precision).
Robustness
Robustness of the method was carried out by making slight deliberate changes in the analytical methodology like flow rate and wavelength. It was observed that there were no changes in system suitability parameters like USP tailing factor, theoretical plates and resolution, which demonstrated that the developed HPLC method is robust.
Limit of Detection (LOD) and Limit of Quantification (LOQ)
The Limit of Detection (LOD) is the lowest amount of analyte in the drug, which can be detected, but not necessarily quantified. The Limit of Quantification (LOQ) is the lowest amount of analyte in the drug, which can be quantitatively determined with suitable precision and accuracy. The LOQ and LOD were determined based on the slope and the standard deviation of the response using the signal-to-noise ratio (S/N) as per ICH Guidelines Q2(R1) 2005.
Degradation studies
According to the ICH guidelines ‘Stability testing of new drug substances and products’ needs that stress testing be carried out to clarify the inherent stability characteristics of the active substance. The main aim of this work was to carry out the stress degradation studies on the Lamivudine, Zidovudine and Nevirapine using the proposed method.
Hydrolytic degradation under acidic conditions
Pipette 3 mL from a standard stock solution containing 0.15 mg/mL, 0.3 mg/mL and 0.2 mg/mL of Lamivudine, Zidovudine and Nevirapine into a 10 mL flask and added 1.0 mL of 0.1 N HCl. Then, the volumetric flask was kept at room temperature for 6 hrs and then neutralized with 0.1 N NaOH and filled with diluents up to the mark. By using 0.45 microns syringe filters, filter the solution and place in vials.
Hydrolytic degradation under alkaline conditions
Pipette 3 mL from standard stock solution containing 0.15 mg/mL, 0.3 mg/mL and 0.2 mg/mL of Lamivudine, Zidovudine and Nevirapine into a 10 mL flask and added 1 mL of 0.1 N NaOH. Then, the volumetric flask was kept at room temperature for 6 hs and then neutralized with 0.1 N HCl and filled with diluents up to the mark. By using 0.45 microns syringe filters, filter the solution and place in vials.
Thermal induced degradation
The sample was taken in a petri dish and kept in a hot air oven at 105ºC for 48 hrs. Then the sample was taken and diluted with diluents to prepare 45 μg/mL, 90 μg/mL and 60 μg/mL of Lamivudine, Zidovudine and Nevirapine were injected into HPLC and it was analyzed.
Oxidative degradation
Pipette 3 mL from a standard stock solution containing 0.15 mg/mL, 0.3 mg/mL and 0.2 mg/mL of Lamivudine, Zidovudine and Nevirapine into a 10 mL flask and added 1 mL of 3% w/v of hydrogen peroxide as oxidation agent filled with diluents up to the mark. That volumetric flask was then kept at room temperature for 30 mins. By using 0.45 microns syringe filters, filter the solution and place in vials.
Conclusion
The proposed RP-HPLC method is accurate, precise, rapid, robust, sensitive and selective for lamivudine, zidovudine and nevirapine. The prescribed method adapted the use of an economical and easily available mobile phase, a UV detector, convenient and easy extraction procedures. And also this method is most suitable for analysis of LC-MS. Washing the column with water and acetonitrile (1:1) ratio and conditioning the column with the mobile phase made it an excellent method for the quantification of lamivudine, zidovudine and nevirapine in bulk drugs and in their pharmaceutical dosage forms. A stability-indicating RP-HPLC method for the estimation of lamivudine, zidovudine and nevirapine in their solid dosage forms was established and validated in accordance with the ICH guidelines. The peak purity data and forced degradation experiment data confirmed that there was no interference of the peaks of the active ingredients with those of any other degradation products or other additives. The developed method can be useful for regular analyses of drugs in bulk and in different formulations without any interference of excipients.
Acknowledgment
We thank Department of Chemistry, Gitam Institute of Technology, Gitam University, Visakhapatnam for providing the necessary facilities.
References
[2] K.A. Sepkowitz, New. Engl. J. Med., 2001, 344, 1764.
[3] R.A. Weiss, “How does HIV cause AIDS?” Science., 1993, 260, 1273.
[4] F.J. Palella, K.M. Delaney, A.C. Moorman, New. Engl. J. Med., 1998, 338, 853.
[5] J.G. Bartlett, US Department of Health and Human Services., 2006.
[6] J.B. Alimonti, T.B. Ball, K.R. Fowke, J. Gen. Virol., 2003, 84, 649.
[7] P.G. Yeni, S.M. Hammer, C.C. Carpenter, D.A. Cooper, M.A. Fischl, J.M. Gatell, B.G. Gazzard, M.S. Hirsch, D.M. Jacobsen, D.A. Katzenstein, J.S. Montaner, D.D. Richman, JAMA., 2002, 288, 222.
[8] http://www.aidsinfo.nih.gov/guidelines/adultAA 111003.pdf
[9] N. Kumarasamy, S. Solomon, S.K. Chatguru, A.J. Cecelia, S. Vallabhaneni, T.P. Flanigan, K.H. Mayer, Clin. Infect. Dis., 2005, 41, 1525.
[10] http://whqlibdoc.who.int/hq/2005/a87017_eng.pdf.
[11] E. De Clercq, Biochem. Pharmacol., 1994, 47, 155.
[12] R. Yarchoan, H. Mitsuya, S. Broder. AIDS therapies. Sci. Am., 1988, 259, 10.
[13] S.S. Patel, P. Benfield, Clin. Immunother., 1996, 6, 307.
[14] A. Gras, S.S. Schneider, J.C. Karasi, A.M. Ternes, N. Sauvageot, C. Karasi-Omes, A.P. Henry, J.C. Schmit, C. Seguin-Devaux, V. Arendt, Curr. HIV Res., 2011, 9, 223.
[15] Z. Li, C. Ding, Q. Ge, Z. Zhou, X. Zhi, X. Liu, Biomed. Chromatogr., 2010, 24, 926.
[16] M. Malm, S. Römsing, C. Obua, Y. Bergqvist, J. Chromatogr. Sci., 2009, 47, 855.
[17] J. Donnerer, B.J. Haas, H.H. Kessler, Pharmacol., 2008, 82, 287.
[18] H. Rebiere, B. Mazel, C. Civade, P.A. Bonnet, J. Chromatogr. B: Analyt. Technol. Biomed. Life. Sci., 2007, 850, 376.
[19] B.P. Vandana, R.P. Parag, D.D. Bhavesh, P. Shivprakash, B. Deepa, Mayank, J. Pharm. Res., 2010, 3, 2322.
[20] Li. Zhou, D. Cungang, Ge. Qinghua, Z. Zhen, L. Xiaofen, Chin. J. Pharm., 2010, 3.
[21] M.M. Krishna, P.N. Rao, I.J. Kumar, L. Burugula, Rao JVLN Seshagiri, Acta. Pharm. Sin. B., 2012, 2, 472.
[22] S. Notari, C. Mancone, M. Tripodi, P. Narciso, M. Fasano, P. Ascenzi, J. Chromatogr. B., 2006, 833, 109.
[23] S. Notari, C. Mancone, G. Ippolito, P. Narciso, L.P. Pucillo, G. Tossini, R.P. Donnorso, F. Gasparrini, P. Ascenzi, J. Chromatogr. B., 2006, 831, 258.
[24] V. Bezy, P. Morin, P. Couerbe, G. Leleu, L. Agrofoglio, J. Chromatogr. B., 2005, 821, 132.
[25] Y. Alnouti, S.R. Lewis, C.A. White, M.A. Bartlett, Rapid. Commun. Mass. Spectrom., 2005, 19, 503.
[26] C.P.W.G.M. Verweij-van Wissen, R.E. Aarnoutse, D.M. Burger, J. Chromatogr. B, 2005, 816, 121.
[27] S. Compain, D. Schlemmer, M. Levi, A. Pruvost, C. Goujard, J. Grassi, H. Benech, J. Mass. Spectrom., 2005, 40, 9.
[28] Y. Alnouti, C.A. White, M.A. Bartlett, Biomed. Chromatogr., 2004, 18, 641.
[29] N.K. Rezk, R.R. Tidwell, A.D.M. Kashuba, J. Chromatogr., 2004, 805, 241.
[30] N.K. Rezk, R.R. Tidwell, A.D.M. Kashuba, J. Chromatogr., 2003, 791, 137.
[31] B.S. Kappelhoff, H. Rosing, A.D.R. Huitema, J.H. Beijnen, J. Chromatogr. B., 2003, 792, 353.
[32] K. Sandhya, N. Nagi Reddy, Raja Manohar, P. Rajashekar, Indo. Am. J. Pharamcit. Res., 2014, 4, 2133.



