Research Article - Der Pharma Chemica ( 2022) Volume 14, Issue 5
Synthesis and Anticonvulsant Activity of Thiadiazoles Derivatives
Shweta Verma* and Asif AliShweta Verma, Faculty of Pharmacy, IFTM University, Lodhipur, Moradabad (UP) 244001, India, Email: shweta_iftm@yahoo.in
Received: 07-May-2022, Manuscript No. dpc-22-63383; Editor assigned: 09-May-2022, Pre QC No. dpc-22-63383; Reviewed: 21-May-2022, QC No. dpc-22-63383; Revised: 24-May-2022, Manuscript No. dpc-22-63383; Published: 31-May-2022, DOI: 10.4172/0975-413X.14.5.34-39
Abstract
Background: The derivatives of Thiadiazoles coupled with various benzaldehyde were synthesized. The synthesized derivatives were evaluated for different physicochemical parameters along with Log P values using different software programs to discover its ability to cross the blood brain barrier. The pharmacological activities such as anticonvulsant activity was evaluated by using PTZ method.
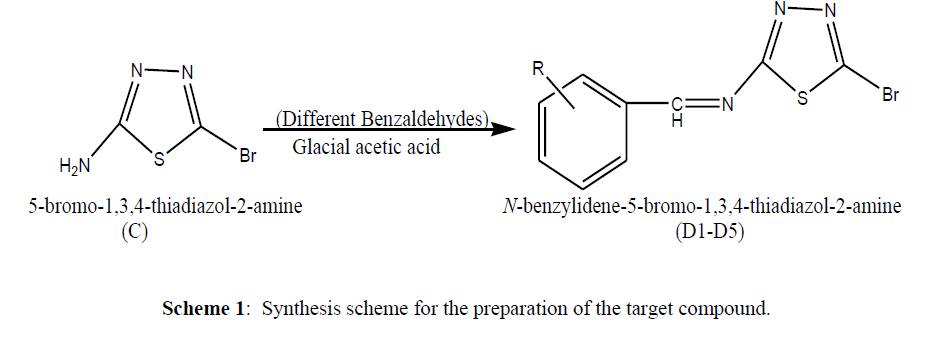
Objective: The new Thiadiazole derivatives were synthesized and assessed for different properties to predict its CNS activity. Thiosemicarbazide was used as a starting material which undergoes cyclization and then bromination to form the intermediate (C). The amino group of intermediates was allowed to react with different benzaldehydes to form the final product (D1-D5). The derivatives were evaluated for the anticonvulsant activity by using PTZ method.
Method: The starting reagent undergoes cyclization to form the product (B) which further undergoes bromination to form the intermediate (C). The intermediate then undergoes final step to form the derivatives in which amino group of intermediates was allowed to react with different benzaldehydes. The reactions of all the synthesized compounds were checked by TLC; final derivatives were purified by recrystallization and characterized by different spectroscopic methods. The similarity calculation and various physicochemical parameters were evaluated by using different software’s and then the derivatives were evaluated for anticonvulsant activity.
Result: The number of derivatives of Thiadiazole was synthesized and various physicochemical parameters were optimized which reveal that the compound containing methoxy group such as C2 and C5 exhibited significant potential when compared with the standard drug Sodium Valproate.
Conclusion: In conclusion, we have synthesized, evaluated various physicochemical parameters and anticonvulsant activity of new thiadiazole derivatives. The various derivatives were successfully synthesized and characterized by spectroscopic methods. It was identified that some derivatives have mild to moderate anticonvulsant activity. Nevertheless, the synthesis of these agents might be valuable in the future research and development for the development of the CNS active agents..
Keywords
Thiadiazole; Physicochemical Parameters; CNS activity; Blood brain barrier; Anticonvulsant; PTZ method
Introduction
Thiadiazoles compound are the heterocyclic in structure comprising of five-membered nitrogen–sulfur ring system with two nitrogen and one sulfur atom and are broadly present as an important structure in various important active molecules which have medicinal properties. The thiadiazols van also becomes an intermediate in the synthesis of number of other significant medicinal compounds [ 1 ]. The different isomeric forms of the thiadiazole such as 1, 2, 3-thiadiazole; 1, 2, 5-thiadiazole; 1, 2, 4-thiadiazole and 1, 3, 4-thiadiazole are found to exist with number of potent activities [2]. The ring contains sulfide linkage, which gives increase activity of thiadiazole; and the physical and chemical properties of thiadiazole can be affected by many other atoms present in the structure such as nitrogen, oxygen and sulfur atoms [3]. Many important medicinal agents such as anti-inflammatory, antimicrobial, antiviral, antiepileptic, antitubercular possess thiadiazol nucleus as a core structure in it. Therefore, thiadiazole is considered to be important scaffold which consists of potent activities [4].
In the present work described here, we carried out the synthesis of new thiadiazole derivatives from thiosemicarbazide using and the structures of the derivatives were confirmed based on spectral data. Additionally, the derivatives were tested for their anticonvulsant activity and various other important parameters too.
Materials and Methods
EXPERIMENTAL
All the chemicals were procured from Sigma-Aldrich and were used without further purification. Melting points were determined by open capillary tubes on Electrothermal capillary apparatus, the results obtained are uncorrected. IR spectra of the derivatives were obtained on potassium bromide plates on Schimadzu FTIR Spectrophotometer 8300. 1HNMR spectra of the derivatives were recorded on a Bruker DRX-300 spectrophotometer and 13C-NMR spectr
Synthesis and characterization of compounds
Step I: Synthesis of 5-bromo-1, 3, 4-thiadiazol-2-amine
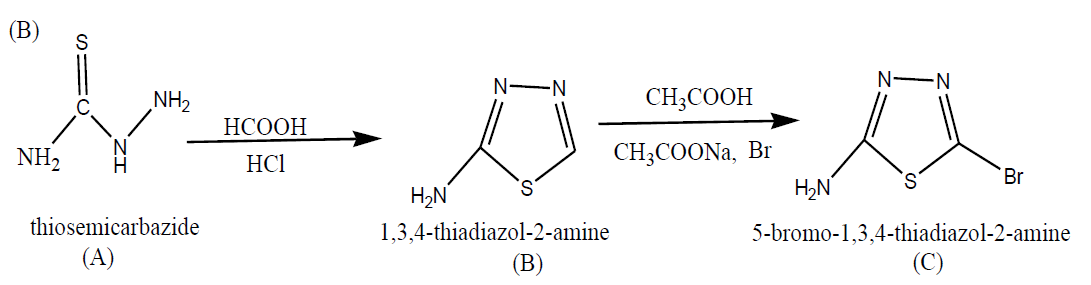
Synthesis of 5-bromo-1, 3, 4-thiadiazol-2-amine (B)
20 ml of formic acid was taken in 250 ml of round bottom flask and 10 gm of thiosemicarbazide was dissolved into it. The mixture of the above two was stirred for 1 hr. at room temperature. After completion of 1hr the reaction mixture was cooled and to this cooled solution 5ml of HCL was added drop by drop. The reaction was monitored by TLC for identifying the completion of reaction. After that the mixture was allowed to cool at 0ºC and basified with ammonium hydroxide solution. The off-white solid product was obtained which was filtered, washed with water and then dried.
Physical paremeters
Percentage yield : 73.49%
Melting range : 83-185ºC
Rf value : 0.78
Mobile phase : n- hexane: Ethyl acetate (1.5:3)
Molecular formula : C2H2N3S
General method for the synthesis of Compound (C)
To a stirred solution of B, (4.0 g) sodium acetate (16 g) in 50ml of acetic acid at 10ºC, bromine was added drop by drop (8.1 g). The mixture was stirred for 3 hours at room temperature. The saturated sodium bicarbonate solution was added for basifying the mixture. The final obtained compound was further extracted with ethyl acetate and the two layers get separated, washed with water and dried over sodium sulphate.
D1: N-(4-nitrobenzylidene)-5-bromo-1,3,4-thiadiazol-2-amine
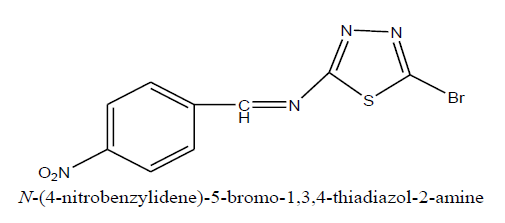
Physical paremeters
Percentage yield : 68.53%
Melting range : 168-171ºC
Rf value : 0.83
Mobile phase : n- hexane: Ethyl acetate (1.5:3)
Molecular formula : C2H2BrN3S
Characterization: IR (KBr, cm-1): 3240 (str, NH amino), 3050 (str, CH), 1640 (C=O amide), 1230 (C-N arom), 600 (str, C-Br), 745 (str, C-C arom).1NMR (300 MHz; DMSO)δppm: 4.21 (s, C-NH)
Step II: Synthesis of target compounds (N-(substituted benzylidene)-5-bromo-1, 3, 4-thiadiazol-2-amine)
General Procedure for the synthesis of N-(substituted benzylidene)-5-bromo-1, 3, 4-thiadiazol-2-amine (D1-D5)
0.5gm of compound (C) was dissolved in 10 ml of methanol in 250 round bottom flask and equivalent molar of different benzaldehydes was dissolved in smallest amount of methanol and the mixture was heated at 20-25ºC for 3 hr with a pinch of glacial acetic acid. The completion of reaction was monitored by TLC. After the completion of reaction, mixture was allowed to cool. The product was precipitated from the cold solution. Filter off the precipitate and recrystallized with ethanol (Table 1).
| Cpd. no. | Cpd code. | R |
|---|---|---|
| 1 | D1 | 4-NO2 |
| 2 | D2 | 4-OCH3 |
| 3 | D3 | 3,4,5-OCH3 |
| 4 | D4 | 3, 4-OCH3 |
D1: N-(4-nitrobenzylidene)-5-bromo-1,3,4-thiadiazol-2-amine
Percentage yield : 61.32 %
Melting range : 158-161ºC
Rf value : 0.69
Mobile phase : n- hexane: Ethyl acetate: (1:2)
Molecular formula : C10H3BrN3OS
Characterization
IR (KBr, cm-1): 3060 (str, CH arom), 2921 (str, CH alip), 1230 (str, C-N arom), 1236 (str, C-N alip), 740 (str,C-C arom), 1510 (str,C=C arom). 1H NMR (300 MHz; DMSO) δppm: 7.0-7.6 (4-H, Ar-H); 8.1 (s, 1H, CH)
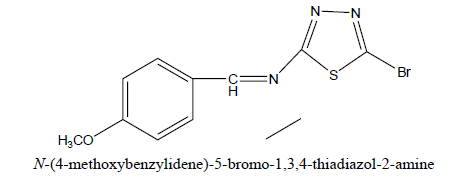
D2: N-(4-methoxybenzylidene)-5-bromo-1, 3, 4-thiadiazol-2-amine
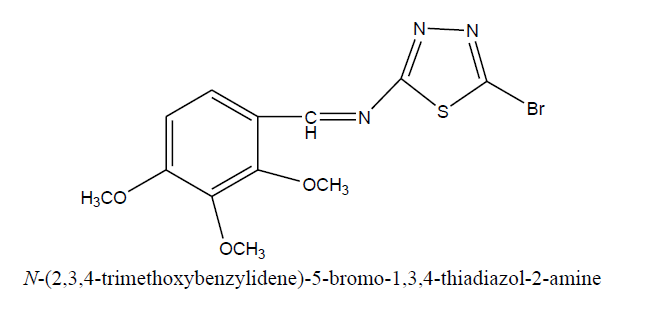
Physical parameters
Percentage yield : 53.03 %
Melting range : 155-157ºC
Rf value : 0.71
Mobile phase : n- hexane: Ethyl acetate: (1:2)
Molecular formula : C9H5BrN4O2S
Characterization
IR (KBr, cm-1): 3062 (str, CH arom), 2917 (str, CH alip), 1231 (str, C-N arom), 1233 (str, C-N alip), 741 (str,C-C arom), 1510 (str,C=C arom). 1H NMR (300 MHz; DMSO) δppm: 7.1-7.6 (4-H, Ar-H); 8.6 (s, 1H, CH); 3.74(s, 3H, o-OCH3); 3.77 (s, 3H, m-OCH3); 3.85(s, 3H, p-OCH3).
D4: N-(3,4-dimethoxybenzylidene)-5-bromo-1,3,4-thiadiazol-2-amine
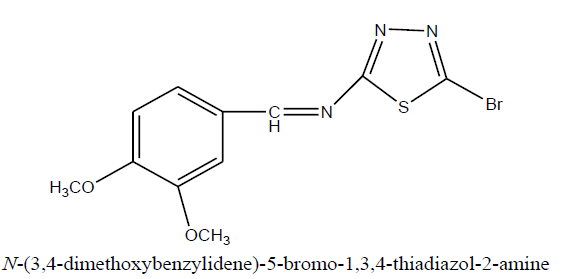
Physical parameters
Percentage yield : 53.03 %
Melting range : 179-181ºC
Rf value : 0.61
Mobile phase : n- hexane: Ethyl acetate: (1:2)
Molecular formula : C11H0BrN3O2S
Characterization
IR (KBr, cm-1): 3065 (str, CH arom), 2911 (str, CH alip), 1232 (str, C-N arom), 1237 (str, C-N alip), 743 (str,C-C arom), 1512 (str,C=C arom). 1H NMR (300 MHz; DMSO) δppm: 7.2-7.9 (4-H, Ar-H); 8.6 (s, 1H, CH); 2.51 (s, 3H, m-OCH3); 3.32(s, 3H, p-OCH3).
Similarity Calculation
The similarity parameter of the synthesized derivatives with respect to standard drug and the physicochemical similarity was calculated from a set of 7 physicochemical properties. The calculation was done by using software programs and the observed data was shown in Table 2.
| Cpd. Code | Similarity,b (in %) to Sodium Valproate |
|---|---|
| D1 | 50 |
| D2 | 85 |
| D3 | 73 |
| D4 | 79 |
The derivatives formed shows slight to moderate similarity with respect to standard drug. The similarity varies from 50-85% was observed.
a (1 - R) X 100 where R = quadratic mean (root mean square mean). b physicochemical properties used for calculation: Molecular weight; Molar refractivity; Connolly solvent accessible surface area; Connolly molecular surface area; Connolly solvent excluded volume; Topological polar surface area; Molecular topological index.
Evaluation of Physicochemical parameters
The aim for the synthesis of the drug is to enhance the CNS potency in order to cross the blood brain barrier (BBB). Lipophilicity was considered to be one of the most important parameters for this property and can be calculated from the partition coefficient. Partition coefficient can be calculated by shake-flask method and by different computational methods. The log P values of all the compounds were determined experimentally and through online software’s and were calculated by Moriguchi method. Other parameters like molecular weight, n ON value, n OH NH value, n-violations and number of rotatable bonds are also calculated through various online software’s and is represented in (Table 3).
| Comp. Code | Log P | Â M.W. | Â n-ON | Â Â n-OHNH | Â n-violation | Rotatable Bonds |
|---|---|---|---|---|---|---|
| D1 | 2.48 | 313.13 | 6 | 2 | 0 | 5 |
| D2 | 2.75 | 296.96 | 6 | 2 | 0 | 5 |
| D3 | 2.54 | 356.98 | 6 | 2 | 0 | 5 |
| D4 | 2.01 | 328.19 | 7 | 2 | 0 | 6 |
Pharmacological Evaluation
Anticonvulsant activity
The anticonvulsant activity was performed by PTZ method. Prior permission of the Animal Ethics Committee was obtained and all experiments were conducted according to the approved protocol. Pentylenetetrazol was employed as a standard (positive control). Statistical analysis of the results in the test group was done by comparison with the results in the control group employing one way ANOVA. Level of significance was fixed at p<0.05.
Anticonvulsant Activity by PTZ method
Anticonvulsant activity of the target compounds were carried out by PTZ method. The permission from Animal Ethics Committee was obtained priorly and the tests were conducted according to the approved protocol (837/PO/Re/S//04/CPCSEA). All the solutions were prepared freshly on examination day and given orally in a volume of 0.5% CMC to 100-150g body weight of rat. The experimental animals were treated PTZ with (60 mg/ kg, n = 6, i. p), or the compounds (200 mg/kg) 60 min before evaluation. The control group was given saline solution
Induction of Epilepsy
Convulsions were induced by an intraperitoneal (i. p.) injection of Pentylenetetrazol (60) mg/Kg body weight) in saline [5].
Administration of Test substance
Each derivative (200mg/Kg body weight) was dissolved in saline administered through i. p. injection for one week prior to the injection of PTZ and the anticonvulsant activity was assessed after 30 min interval of administrations (Table 4).
| Compound code | Dose | Onset of clonic convulsion (min.) | Duration of convulsion (min) | Mortality/ used (%) |
|---|---|---|---|---|
| Control | Vehicle | 0.00±0.10 | 0.00±0.00 | 0/6 (0%) |
| PTZ | 4mg/kg | 8.00±0.10 | 2.50±0.30 | 5/6 (83.34%) |
| D-1 | 5mg/kg | 6.56±0.40 | 0.96±0.44 | 3/6 (50%) |
| D-2 | 5mg/kg | 1.65±1.10*** | 0.57±0.10*** | 0/6 (0%) |
| D-3 | 5mg/kg | 3.70±2.10* | 2.10±2.10* | 1/6 (16.67%) |
| D-4 | 5mg/kg | 3.10±3.30** | 1.77±1.22** | 0/6 (0%) |
Conclusion
Series of Thiadiazole were designed and synthesized using the pathway shown in schemes (1). The synthesized derivatives were evaluated for anticonvulsant and various physicochemical properties along with similarity of the compounds with respect to standard drug sodium valproate. The target compounds (D1-D4) have been synthesized by reaction of thiosemicarbazide with formic acid in the presence of HCl to form (B) which further reacted with sodium acetate in acetic acid in the presence of bromine to form (C). The compound (C) was allowed to react with different substituted benzaldehydes to form the final products. The reactions of all the synthesized compounds were watched by TLC and the derivatives were purified by recrystallization and characterized by various spectroscopic methods.
The values of physicochemical property for the target compounds were found very near to standard drug. The physicochemical similarity of the target compounds was calculated with respect to standard drug Sodium Valproate and shown in Table 2. The target compounds showed mild to moderate structural similarity with respect to standard drug.
The drugs were synthesized in in order to enhance the potency to cross the blood brain barrier (BBB) and designed it to be CNS active. Other parameters like molecular weight, n ON value, n OHNH value, n-violations and number of rotatable bonds are determined through online software molinspiration (Tables 3).
The pharmacological evaluation, anticonvulsant activity for the test compounds were performed. Some of target compounds have shown significant CNS activity. Compound bearing methoxy group in benzaldehyde ring D2, D3 and D4 has shown the significant anticonvulsant activity among which D2 has maximum potency in all the synthesized derivatives. However, the compound having nitro group on ring showed moderate activity. So, in our work we have defined the synthesis, physicochemical studies and evaluation for anticonvulsant activity of some new Thiadiazole derivatives. The synthesized derivatives were characterized by spectroscopic methods such as IR, NMR. The derivatives found to possess mild to moderate anticonvulsant activity. However, further studies of these derivatives might be beneficial in the future research and development of the target compounds for the improvement of the CNS activity.
References
- Serban G, Stanasel O, Serban E, et al., Drug Des Devel Ther. 2018, 12: p. 1545-1566.
- Siddiqui N, Ahuja P, Ahsan W, et al., J Chem Pharm. 2009, 1: p. 19-30.
- Bhat AR, Tazeem Azam A, Choi I, et al., Eur J Med Chem. 2011, 46: p. 3158-3166.
- Jain A. Sharma Simant. Vaidya A, et al., Chem Biol Drug Des. 2013, 81: p. 557-576.
- Ray SK, Poddar MK. Biochem Pharmacol. 1985, 34: p. 553-557.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref