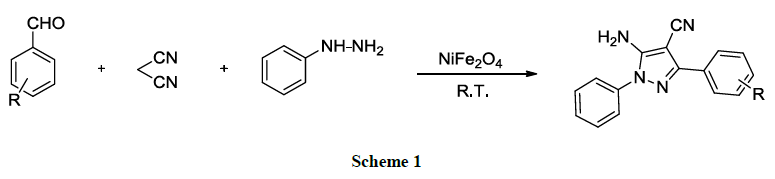
Research Article - Der Pharma Chemica ( 2018) Volume 10, Issue 4
Synthesis of Polysubstituted Amino Pyrazole via Multicomponenet Strategy Using NiFe2O4 Nanocatalyst
Vijay V Dabholkar*, Swapnil K Kurade, Keshav S Badhe, Krishnan Karthik and Shrikant Anpat
Organic Research Laboratory, Department of Chemistry, Guru Nanak College, G.T.B Nagar, Mumbai-400 037, India
- *Corresponding Author:
- Vijay V Dabholkar
Organic Research Laboratory
Department of Chemistry
Guru Nanak College
G.T.B Nagar, Mumbai-400 037, India
Abstract
A NiFe2O4 nanoparticle found to be excellent catalyst for multicomponent synthesis of 5-amino-1H-pyrazole-4-carbonitrile using substituted aromatic aldehyde, malononitrile and phenyl hydrazine at room temperature. The important features of this process are short reaction time, easy work-up, purification by a non-chromatographic method.
Keywords
Amino pyrazole, Aromatic aldehyde, Nanoparticle, Multicomponent, Nickel ferrite
Introduction
In recent years, MCR’s are one of the most important methods for synthesis of structurally complex and highly functionalized molecules by reaction of three or more reactant in a single step. These one-pot methodologies have several advantages over conventional stepwise reaction strategies by saving energy, reducing wastage, short reaction time and avoiding protection and deprotection of various functional groups in reactants [1,2].
Heterocyclic chemistry is very important topic in the Pharmaceutical chemistry and Organic Chemistry. Among the various heterocyclic molecules discovered and developed, the nitrogen containing compound show the great application in biology and chemistry. nA heterocyclic compound containing aza group as a functional group, the pyrazoles have tremendous application in pharmaceutical and agrochemical industries [3-5]. These compounds are known to show antidepressant [6], analgesic [7], antimalarial [8], antitumor [9], antifungal [10] and antimicrobial [12]. From a reported study on pyrazole derivatives revealed its application in pesticide as an herbicidal [13] and insecticidal [14] activities.
Pyrazole derivative has been synthesized by various methods [15-17]. Pyrazole derivative synthesis was reported using The reaction between 1,3-dipolar cycloaddition of the diazo compound onto triple bond [18]. Several drawbacks such as anhydrous condition, expensive catalyst, long reaction time, tedious work-up observed for these reported methods. Consequently, great effort has been made to find efficient synthesis process via nanocatalysis [19].
NiFe2O4 nanoparticle kind of soft magnetic particle has much application such as catalysis [20], microwave devices [21] etc. By considering importance of nanocatalyst in organic synthesis here in we demonstrate NiFe2O4 catalyzed synthesis of 5-amino-1H-pyrazole-4-carbonitrile at room temperature.
Materials and Methods
Chemicals and analysis
All chemicals used without purification and procured from S.D. Fine Ltd. Mumbai, India. All synthesized compounds for melting points were determined in open capillary tubes in an electro thermal apparatus. Thin layer chromatography on silica gel coated aluminium plates (Merck) as adsorbent and UV light as the visualizing agent used to check the compound purity. FT-IR Spectra for compounds were recorded on Bruker Spectrometer in the region of 400-4000 cm-1. 1H and 13C-NMR spectra were recorded on Varian 500 MHz NMR spectrophotometer using CDCl3/DMSO-d6 as solvent and TMS as an internal standard (Chemical shifts in δ ppm). Powder X-ray diffraction analysis of catalyst was collected with monochromatic Cu Kα radiation (λ=1.54059 Å) at 40 kV and 15 mA using Shimadzu 7000S diffractometer.
General procedure for synthesis of NiFe2O4
NiFe2O4 nanoparticles were synthesized by co-precipitation method [24]. Nickel nitrate, ferric nitrate and sodium hydroxide were used as starting materials. Aqueous solutions of ferric nitrate and nickel nitrate were prepared in de-ionized water; NaOH solution was then added to it slowly and stirred continuously using a magnetic stirrer until a pH reached to 10-11. This solution was then heated at 80°C for an hour. The obtained precipitate was thoroughly washed with distilled water till pH of filtrate become 7. It was kept overnight for drying. The obtained powder was grounded and calcined at temperatures 500°C for 3 h.
General procedure for the synthesis of 5-amino-1H-pyrazole-4-carbonitrile
In a 25 ml round bottomed flask, aryl aldehyde (3 mmol), malononitrile (3 mmol) and NiFe2O4 (0.03 g) in ethanol (3 ml) were stirred at room temperature till the reactants were fully consumed (TLC). To the solution phenyl hydrazine (3 mmol) was added and the reaction mixture was stirred at ambient temperature (29°C) till the complete consumption of aldehyde (TLC). The solid catalyst was recovered by a magnet. The removal of solvent resulted in the recovery of solid product. The product was recrystallized using ethanol. Purified product characterized by melting point, NMR and IR.
Results and Discussion
Optimal condition (Scheme 1) for the modal reaction of malononitrile, phenyl hydrazine and substituted aromatic aldehyde was carried out using NiFe2O4 nanoparticle as a heterogeneous catalyst.
Spectral data
5-Amino-1,3-diphenyl-1H-pyrazole-4-carbonitrile (Entry No. 1): IR spectra: (KBr) [cm-1]: 3490 cm-1 & 3350 (NH2), 2359 (C≡N), 1610 (C=N); 1H-NMR interpretation: (500 MHz, DMSO-d6): δ(ppm)=6.742-7.867 (m, 10H, Ar-H), 10.333 (s, H, NH2); 13C-NMR interpretation: (500 MHz, DMSO-d6): δ(ppm)=112.462 (C-CN), 119.194 (CN), 126.055-136.320 (C=C, ArC), 145.784 (C=N).
5-Amino-3-(3-nitrophenyl)-1-phenyl-1H-pyrazole-4-carbonitrile (Entry No. 2): IR spectra: [cm-1]: 3470 cm-1 & 3360 (NH2), 2362 (C≡N), 1620 (C=N); 1H-NMR interpretation: (500 MHz, DMSO-d6): δ(ppm)= 8.478 (s, 2H, NH2), 6.970-8.159 (m, 9H, Ar-H); 13C-NMR interpretation: (500 MHz, DMSO-d6): δ(ppm)= 112.440 (C-CN), 121.098 (CN), 129.510-144.610 (C=C, ArC), 149.157 (C=N).
Firstly, we conducted the reaction in one pot between all the reactants using NiFe2O4 nanoparticle as a catalyst results in the formation of the product along with some by product To encounter this problem, we attempt reaction using sequential addition of the reagent that leads to the product with excellent yield along with a reduction in the amount of byproducts. Firstly, we carried out the reaction between malononitrile and benzaldehyde in presence of NiFe2O4 which leads to the formation of benzylidene malononitrile as an intermediate. The intermediate has a highly polarized double bond, to which phenyl hydrazine added under the same conditions results in the desired product. Knoevenagel reaction between aromatic aldehyde and malononitrile followed by a Michael addition intermolecular cyclization gives desired product formation.
To find out optimal solvent, the reaction was performed in different protic and aprotic solvents such as water, ethanol, DMF, DMSO. Ethanol found to be appropriate solvent as better results obtained (Table 1).
| Entry | Solvent | Yield of product (%) |
|---|---|---|
| 1 | H2O | 79 |
| 2 | EtOH | 94 |
| 3 | MeOH | 86 |
| 4 | DMF | 34 |
| 5 | DMSO | 42 |
Reaction conditions: Benzaldehyde (3 mmol), Malononitrile (3 mmol), Phenyl hydrazine (3 mmol), solvent (3 ml), NiFe2O4 (0.03 g), ambient temperature (29°C)
Table 1: Solvent Selection; The reaction of benzaldehyde, malononitrile, and phenyl hydrazine in the presence of NiFe2O4 in different solvents at ambient temperature 29°C
In search of the quantity of catalyst required for the reaction and we observed that yield of the product were affected by catalyst loading (Table 2). We have carried out the reaction with catalyst loading from 0.01 to 0.05 g. We found that catalyst loading of 0.03 g gives better yield. Further, any increase in catalyst loading did not show the significant change in yield.
| Entry | Catalyst quantity (g) | Yield of product (%) |
|---|---|---|
| 1 | 0.01 | 70 |
| 2 | 0.02 | 94 |
| 3 | 0.03 | 94 |
| 4 | 0.04 | 94 |
| 5 | 0.05 | 94 |
Reaction conditions: Benzaldehyde (3 mmol), Malononitrile (3 mmol), Phenyl hydrazine (3 mmol), EtOH (3 ml), ambient temperature (29°C)
Table 2: Catalyst quantity; The reaction of benzaldehyde, malononitrile, and phenyl hydrazine using different quantity of NiFe2O4 in EtOH at ambient temperature
As heterogeneous catalyst, recovery and reusability of the catalyst is a very important factor. So, NiFe2O4 can be used a number of times without the significant change in catalytic activity. To examine the reusability of catalyst we run the reaction using recovered catalyst of the first run for four times (Table 3). It did not show a much more significant loss of catalytic activity.
| Run | Yield of product (%) |
|---|---|
| 1 | 94 |
| 2 | 94 |
| 3 | 94 |
| 4 | 93 |
| 5 | 93 |
Reaction conditions: aldehyde (3 mmol), malononitrile (3 mmol), phenyl hydrazine (3 mmol), EtOH (3 ml), NiFe2O4 (0.03 g), reaction temperature (29°C).
Table 3: Reusability of the NiFe2O4 catalyst
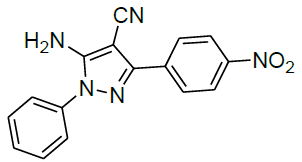
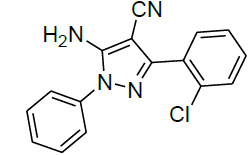
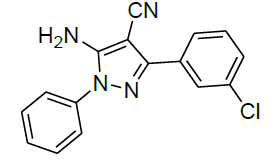
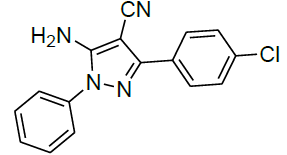
Under all the optimized reaction parameter, different substituted aromatic aldehydes were reacted with malononitrile and phenyl hydrazine to obtain the corresponding 5-amino-1H-pyrazole-4-carbonitrile in high yield (Table 4). It was found that aromatic aldehyde with electron withdrawing group offers an excellent yield of the product as compared to the aromatic aldehyde with electron donating group.
| S. No. | Substituted benzaldehyde | Product | Yield (%) |
Time (min) |
M.P. (°C) | Reported M.P. (°C) |
|---|---|---|---|---|---|---|
| 1 | 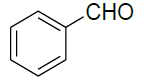 |
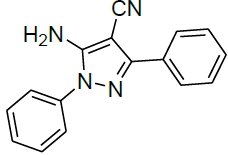 |
96 | 8 | 159-160 | 160−161[22] |
| 2 | 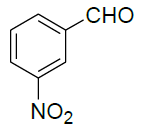 |
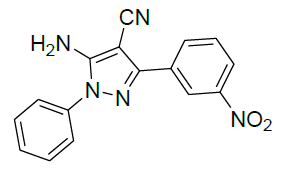 |
97 | 5 | 128-130 | 129–130[22] |
| 3 | 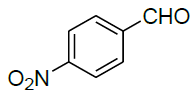 |
 |
98 | 5 | 164-160 | 164–165[22] |
| 4 | 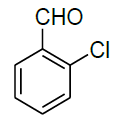 |
 |
96 | 6 | 50-52 | |
| 5 | 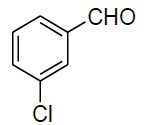 |
 |
94 | 8 | 113-114 | |
| 6 | 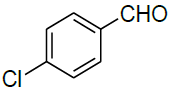 |
 |
97 | 6 | 128-130 | 129–130[22] |
| 7 | 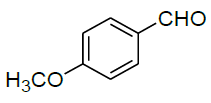 |
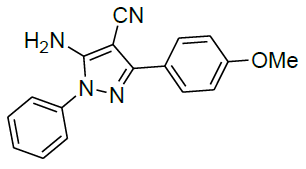 |
96 | 7 | 106-108 | - |
| 8 | 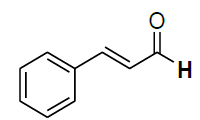 |
 |
95 | 10 | 152-154 | - |
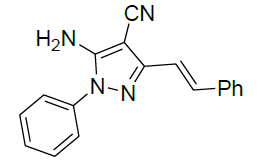
| 9 | 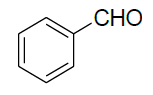 |
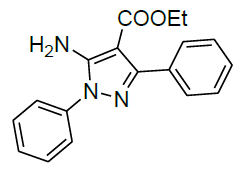 |
96 | 9 | 142-144 | - |
| 10 | 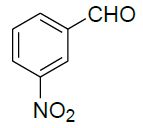 |
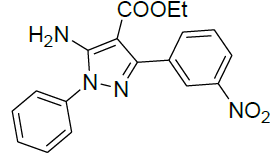 |
98 | 7 | 96-98 | - |
| 11 | 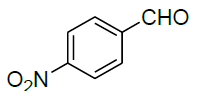 |
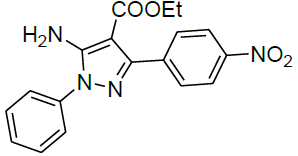 |
99 | 6 | 125-127 | - |
| 12 | 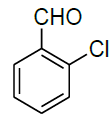 |
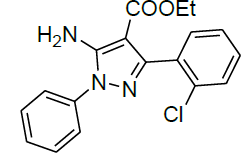 |
98 | 8 | 78-80 | - |
| 13 | 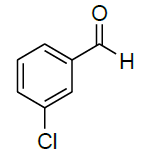 |
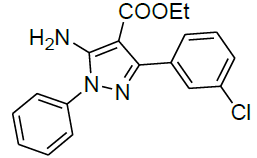 |
95 | 10 | 117-119 | - |
| 14 | 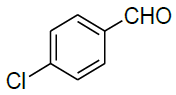 |
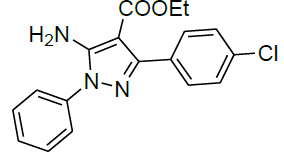 |
99 | 8 | 120-122 | - |
| 15 | 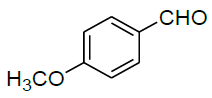 |
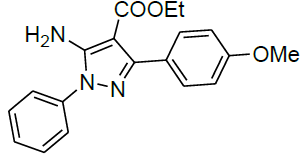 |
97 | 10 | 112-113 | - |
| 16 | 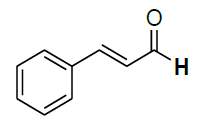 |
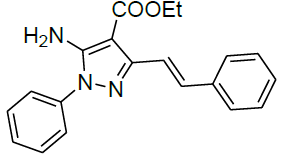 |
93 | 12 | 155-157 | - |
Reaction conditions: Aldehyde (3 mmol), Malononitrile (3 mmol), Phenylhydrazine (3 mmol), EtOH (3 ml), NiFe2O4 (0.03 g), reaction temperature (29°C)
Table 4: Synthesis of 5-amino-1H-pyrazole-4-carbonitrile using NiFe2O4 nanocatalyst
Characterization of catalyst
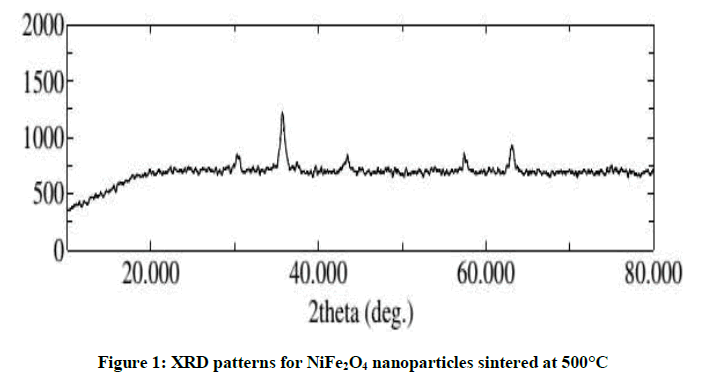
The structural characterization of NiFe2O4 nanoparticles was done by X-ray Diffraction using CuKα radiation (λ=1.54059 Å) at 40 kV and 15 mA shown in Figure 1. The XRD patterns show the formation of single phase inverse cubic spinal nickel ferrite (the XRD peaks were compared to the standard PDF card number 742081 for inverse cubic nickel ferrite).
Morphological analysis
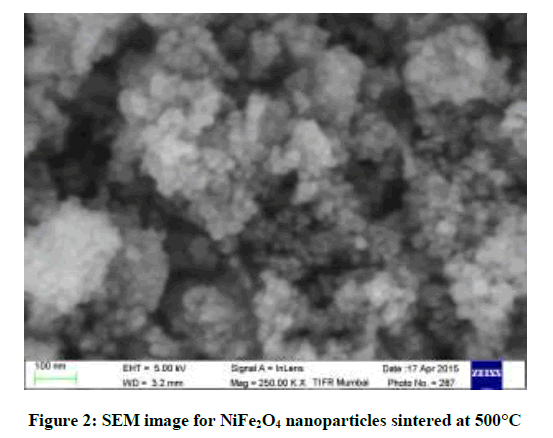
The morphology of NiFe2O4 nanoparticles was studied using SEM shown in Figure 2. Most of the particles are found to be spherical in shape.
Conclusion
A NiFe2O4 nanocatalyst act as efficient catalyst for synthesis of 5-amino-1H-pyrazole-4-carbonitrile by using three component one pot protocols at room temperature. This process is simple fast and yield of product is excellent. This process has key features such as environmentally green and friendly, inexpensive.
Acknowledgement
The Authors are thankful to the principal and Management of Guru Nanak college of Arts, Science & Commerce, Sion (E) for constant encouragement and providing necessary facilities. Authors are also thankful to TIFR, Mumbai for providing spectral data.
References
- H. Bienayme, C. Hulme, G. Oddon, P. Schmitt, Chem. Eur. J., 2000,6, 3321.
- S.F. Oliver, C. Abell, Curr. Opin. Chem. Biol., 1999, 31, 299.
- A. Hasaninejad, A. Zare, M. Shekouhy, J. Comb. Chem., 2010, 12, 844-849.
- A. Hasaninejad, A. Zare, M. Shekouhy, Green Chem., 2011, 13, 958-964.
- K. Anzai, M. Furuse, A. Yoshida, A. Matsuyama, T. Moritake, K. Tsuboi, N. Ikota, J. Radiat. Res., 2004, 45, 319-323.
- D.M. Bailey, P.E. Hansen, A.G. Hlavac, E.R. Baizman, J. Pearl, A.F. Defelice, M.E. Feigenson, J. Med. Chem., 1985, 28, 256-260.
- S.A. Gursoy, G. Demirayak, K. Capan, Eur. J. Med. Chem., 2000, 35, 359-364.
- S.B. Katiyar, K. Srivastava, S.K. Purib, P.M.S. Chauhana, Bioorg. Med. Chem. Lett., 2005, 15, 4957-4960.
- G. Daidone, B. Maggio, S. Plescia, D. Raffa, C. Musiu, C. Milia, G. Perra, M.E. Marongiu, Eur. J. Med. Chem., 1998, 33, 375-382.
- Y. Hiyama, K.M. Suzuki, J. Yamagishi, J. Med. Chem., 2004, 47, 3693-3696.
- D. Nauduri, G.B. Reddy, Chem. Pharm. Bull., 1998, 46, 1254-1260.
- F.Q. He, X.H. Liu, B.L. Wang, Z.M. Li, Heterot. Chem., 2008, 19, 21-27.
- J.J. Parlow, J. Heterocycl. Chem., 1998, 35, 1493-1499.
- G. Varvounis, Y. Fiamegos, G. Pilidis, Adv. Heterocycl. Chem., 2004, 87, 141-272.
- A.M. Salaheldin, A.M.F. Oliveira-Campos, L.M. Rodrigues, Tetrahedron Lett.,2007, 48, 8819-8822.
- G. Varvounis, Y. Fiamegos, G. Pilidis, Adv. Heterocycl. Chem., 2007, 95, 27-141.
- R. Martin, M.R. Rivero, S.L. Buchwald, Angew. Chem. Int. Ed. Engl., 2006, 45, 7079-7082.
- V. Polshettiwar, R.S. Varma, Green Chem., 2010, 12, 743-754.
- J. Sloczynski, J. Janas, T. Machej, J. Rynkowski, J. Stoch, Appl. Catal. B.,2000, 24(1), 45-60.
- A. Baykal, Z. Durmus, H. Kavas, M.S. Toprak, Y. Koseoglu, Turk. J. Chem., 2009, 33, 33-45.
- P.S. Bhale, S.B. Dongare, U.B. Chanshetti, Res. J. Chem. Sci., 2014, 4(9), 16-21.
- S. Joshi, M. Kumar, S. Chhoker, G. Srivastava, M. Jewariya, V.N. Singh, J. Mol. Struct., 2014, 1076, 55-62.