Research Article - Der Pharma Chemica ( 2019) Volume 11, Issue 3
UPLC Method for the Quantification of Elvitegravir, Cobicistat, Emtricitabine and Tenofovir Disoproxil Fumarate in Tablets Using OGD Reference Dissolution Medium
Vittal SP, Rao SM and Ramakrishna K*Ramakrishna K, APL Research Centre (A Division of Aurobindo Pharma Limited), Hyderabad-500072, Telangana, India, Tel: +91 9912564884, Email: karipeddirk@gmail.com
Abstract
The current paper discuss about strategic development and validation activity performed for quantification of Elvitegravir (EL), Cobicistat (CO), Emtricitabine (EM) and Tenofovir Disoproxil fumarate (TDF) in a combination drug product using Office of Generic drugs (OGD) recommended Dissolution medium. Ultra Performance Liquid Chromatographic (UPLC) technique method was chosen and developed a method with a run time of four minutes. Mobile phase A consists of 0.1% perchloric acid and Mobile phase B consists of Acetonitrile. Gradient elution technique was opted with an optimized flow rate of 0.3 ml per minutes. Acquity UPLC BEHC18 (100 mm × 2.1 mm ID), 1.7ï particle size column is finalized for testing purpose with a detection wavelength of 260 nm. Typical retention times observed for EM, TDF, CO and EL are 0.89, 1.42, 2.01 and 2.77 min respectively. Method is found to be linear over the specified concentration of 3.71- 44.56 μg/ml of EL, 1.77-21.23 μg/ml for CO, 4.90-58.74 μg/ml for EM and 7.37-88.40 μg/ml for TDF with correlation coefficient more than 0.99. Accuracy of the drugs is found to be more than 90% in proposed OGD medium. Developed method could be useful to quantify the drugs in pharmaceutical quality control and contract research laboratories for dissolution profiles at very fast rate.
Keywords
Elvitegravir, Cobicistat, Emtricitabine, Tenofovir Disoproxilfumarate, Dissolution, RP-UPLC
Introduction
Elvitegravir/Cobicistat/Emtricitabine/ Tenofovir Disoproxilfumarate tablet is available in Fixed Drug Combination (FDC) under the brand name Stribild. In other names, it is also called as QUAD. Stribild is prescription medicine approved by United States Food and Drug administration (USFDA) to treat in adults who have never taken medicines for HIV infection earlier [1-3]. Each tablet contains 150 mg of EL, 150 mg of CO, 200 mg of EM and 300 mg of TDF (equivalent to 245 mg of Tenofovir Disoproxil). EL is a HIV medicine known as integrase inhibitor. Chemical nameis “6-(3-Chloro-2-fluorobenzyl)-1-[(2S)-1-hydroxy-3methylbutan-2-yl]-7-methoxy-4-oxo-1,4-dihydroquinoline-3-carboxylic acid”with a molecular weight of 447.9 and an empirical formula of C23H23ClFNO5. CO is a pharmacokinetic enhancer, which would be useful toincrease the effectiveness of EL. CO has a chemical name of “1,3-thiazol-5-ylmethyl [(2R,5R)-5-{[(2S)-2- [(methyl{[2-(propan-2-yl)-1,3- thiazol-4-yl]methyl}carbamoyl)amino]-4-(morpholin-4-yl)butanoyl]amino}-1,6-diphenylhexan-2-yl]carbamate” with a molecular weight of 776.0 with an empirical formula of C40H53N7O5S2.
EM is a Human Immunodeficiency Virus (HIV) Nucleoside Analog Reverse Transcriptase Inhibitor. The chemical classification of EM is Nucleoside Analog. The mechanism of action of EM is defined as a Nucleoside Reverse Transcriptase Inhibitor. EM forms emtricitabine 5'- triphosphate within the cell by phosphorylation. The action of the metabolite is to inhibit the activity of HIV reverse transcriptase both by competing with the natural substrate deoxycytidine 5'-triphosphate and by incorporation into viral DNA causing a termination of DNA chain elongation. EM has a chemical name of“5-fluoro-1-(2R,5S)-[2-(hydroxymethyl)-1, 3-oxathiolan-5-yl]cytosine”. It is a thio analog of cytidine with (-) enantiomer with a molecular weight of 247.24 and molecular formula of C8H10FN3O3S. TDF is a prodrug and exists as fumaric acid salt form of tenofovir. The chemical category of TDF is a nucleoside reverse transcriptase inhibitor analog of adenosine. It is mainly prescribed to treat not only for HIV and also for hepatitis B virus under chronic conditions in adults in combination with other antiviral therapeutic agents. TDF has a chemical name of “9-[(R)-2[[bis[[(isopropoxycarbonyl)oxy]methoxy] phosphinyl] methoxy] propyl]adenine fumarate (1:1)” with a molecular weight of 635.52 and a molecular formula of C19H30N5O10P • C4H4O4.
Upon survey of different research articles, journals and publications indicate that few HPLC Assay test procedures being present for quantification of EL, CO, EM and TDF in FDC products [4-10]. UPLC method is available to determine the degradants present in this formulation [11-19]. However UPLC test methods are not reported to quantify these drugs in OGD recommended dissolution medium. Since all drugs are having different polarity, it is difficult to fix a common chromatographic method with shorter run time. While performing dissolution profiles during drug product development, it is very difficult to conclude the results if it runs for longer run time. Hence to avoid such practical problems, method was targeted to develop using simple volatile buffer which is compatible with low micron ID columns with RP-UPLC. The advantage of using volatile buffers is to increase longevity of column inspite of repeated number of injections when compared against organic buffers.
Validated test procedure is specific with respective to dissolution medium and placebo. Method validation was performed as per ICH Q3 (d) guidance and found to be suitable for quantification of dissolution profiles required for EL, CO, EM and TDF in FDC product by following dissolution conditions as proposed in Office of Generic Drugs (OGD) for QUAD. 0.01 N HCl with 2% w/w Polysorbate 80 is used as Dissolution medium, USP Apparatus type II (Paddle) with a stirring speed of 100 rpm, proposed dissolution volume is 1000 ml. The specified time points are 5, 10, 15, 20 and 30 minutes respectively. Chemical structures of EL, CO, EM and TDF have been illustrated in Figure 1.
Instrumentation
Waters-Acquity UPLC connected to Binary gradient-pump system which has temperature controller to column compartment with an integrated Auto sampler and Photo diode array detector (PDA). Windows based computer is loaded with Empower 2 software which acts as an interface to monitor output signals. Dissolution profiling was performed using Distek dissolution Apparatus type II system. Acquity Ethylene Bridged Hybrid technology (BEH) C18, (100 × 2.1 mm ID) with 1.7μm column was used.
Chemicals
EL, CO, EM and TDF standards, Stribild tablets from Gilead Sciences, Inc. were taken fromAurobindo Pharma limited. Ultra-pure Acetonitrile, Hydrochloric acid of GR grade and Polysorbate 80 of GR grade are taken from Merck. Ultrapure water is taken from Evoqua water purifier.
Development and optimization of UPLC technique
Aim of the current paper is to reproduce, precise and accurate results when performed for Dissolution profiling at short run time in EL, CO, EM and TDF tablets. QUAD is not official or cited in any compendial monographs. There is no RP-UPLC method being published so far for dissolution profiling test. EL, CO, EM and TDF are having different polarities. The pKa values observed for EL is about 6.6, for CO is about 6.4, for EM is about 2.65 and for TDF is about 3.75 respectively. Structural moieties of EM and TDF are showing amine functional groups which may tend to pose peak tailing due to silanol effects. To avoid this it is preferred to choose acidic mobile phase for development activity.
The OGD recommended dissolution medium contains 2% w/w Polysorbate 80. Hence care must be taken during optimizing chromatographic conditions. Due to viscous nature of the medium, there could be probable chance to accumulate back pressure after repeated number of injections which may reduce the life of the column. Especially in UPLC applications, this practical problem can be addressed in two ways i.e., by keeping column oven temperature on higher side and prefer to use volatile buffers which do not give much column back pressure. By considering these issues, method parameters were optimized accordingly. Perchloric acid is strongly acidic and volatile in nature and also a small ion pair reagent. It completely dissociates in water and provides true ion exchange selectivity when interacted with different drugs especially present in FDC products. Hence for mobile phase preparation purpose 0.1% perchloric acid was selected and considered as Mobile phase A and Acetonitrile was chosen as Mobile phase B. Since drugs are having different polarity, to get shorter run time method it was recommended to prefer gradient elution mode by keeping moderate flow rate at 0.3 ml per min. Column oven temperature was kept beyond 40°C to maintain low back pressure from column.
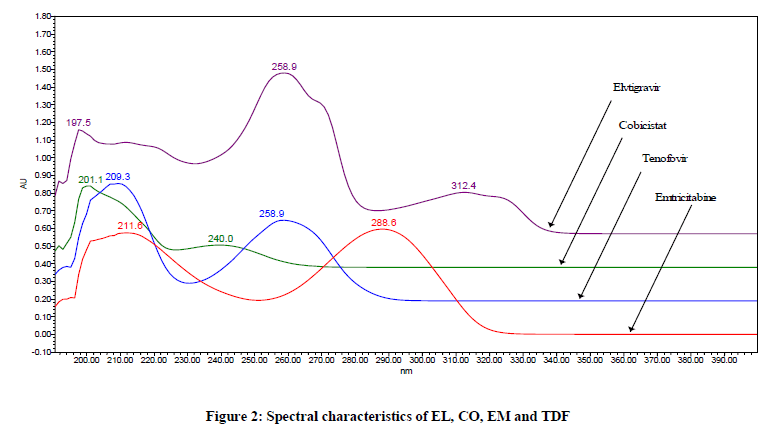
Ethylene bridged hybrid (BEH) technology bonded phase present in Waters Acquity column works on hydrophilic interaction and hence produces a versatile robust separation between the compounds and also can operate at wider usable pH range. Since the buffer used in mobile phase preparation contains acidic pH, it was preferred to keep reverse phase column with C18 chemistry using BEH technology. For trial purpose, Waters Acquity UPLC BEH C18 (100 mm × 2.1 mm id) with 1.7 μm particle size was used and found to be suitable for optimum separation between all drugs present in QUAD. The wavelength maxima observed for EL is about 259 nm, for CO is about 240 nm, for EM is about 288 nm and for TDF is about 260 nm (Spectral data has been mentioned) (Figure 2). To quantity all drug components; a common wavelength of 260 nm has been finalized. Using 2 μl injection volumes for all drugs has shown reproducible area counts which are found to be suitable for drug profiling using 260 nm.
Upon taking several logical gradient trials using 0.1% perchloric acid, Acetonitrile as Mobile phase A and Mobile Phase B, resolution between the drugs is found to be more than 3.0 in the optimized chromatographic conditions. Flow rate was finalized at 0.3 ml per min. Column oven temperature is finalized at 45°C, where column backpressure is found to be under control. In all robustness conditions resolution between each drug is found to be more than 2.5 and USP tailing factor is found to be less than 1.2 for all drugs. This indicates, in optimized chromatographic conditions for quantification of drugs shall not be altered with minor changes that are likely to occur during continuous runs.
Method optimized chromatographic conditions
The finalized chromatographic conditions are given in Table 1. The Typical retention times observed for EM, TDF, CO and EL in the optimized chromatographic conditions are about 0.89, 1.42, 2.01 and 2.77 minutes respectively.
| Column | Waters Acquity UPLC (BEH) C18, 1.7 μ. 100 × 2.1 mm | |||
| Detection | 260 nm (PDA Detector) | |||
| Column temperature | 45°C | |||
| Inj. volume | 2 µL | |||
| Mobile phase A | 1 ml of perchloric acid in 1000 ml of water | |||
| Mobile phase A | Degassed acetonitrile | |||
| Diluent | 10 ml of acetonitrile followed by dissolution medium for preparation of Standard and sample preparation is performed in dissolution medium only. | |||
| Step gradient program | Time (min) | Flow (ml) | % Elution Phase-A | % Elution Phase-B |
| 0 | 0.3 | 60 | 40 | |
| 1.5 | 0.3 | 30 | 70 | |
| 3.2 | 0.3 | 30 | 70 | |
| 3.3 | 0.3 | 60 | 40 | |
| 4 | 0.3 | 60 | 40 | |
Table 1: Chromatographic conditions
| Name of the drug | USP plate count | Tailing factor | Resolution | %RSD for replicate injections |
|---|---|---|---|---|
| EM | 362 | 1.14 | - | 0.5 |
| TDF | 2081 | 1.06 | 3.43 | 0.58 |
| CO | 4201 | 0.94 | 4.73 | 1.33 |
| EL | 7290 | 0.98 | 5.87 | 0.62 |
Table 2: General system suitability data
Preparation of solutions
Preparation of standard solution
Individual Standard stock solution of EL, CO, EM and TDF were prepared at 0.9 mg/ml, 0.9 mg/ml, 1.2 mg/ml and 1.2 mg/ml, initially by dissolving in 10 ml of acetonitrile, further diluted with dissolution medium. This stock solution was diluted to prepare standard solutions at a concentration of 36.0 μg/ml, 48.0 μg/ml, 36.0 μg/ml and 72.0 μg/ml respectively using dissolution medium.
Dissolution test conditions
The dissolution profiling test was conducted for QUAD tablets as per OGD recommended dissolution medium of 2.0% polysorbate 80 in 0.01 N HCl, using USP type II apparatus (Paddle) with 100 rpm stirring speed. Dissolution medium volume is 1000 ml which was maintained at 37°C (± 0.5°C) in dissolution bowls. Samples of about 10 ml were withdrawn from the dissolution bowl at specified time points of 5, 10, 15, 20 and 30 min respectively. After each sampling, about 10 ml of dissolution medium which is maintained at 37°C is placed into each dissolution vessel. Sample solutions are filtered using suitable filters.
Analytical method validation
Stribild tablets are available in FDC with 150 mg of EL, 150 mg of CO, 200 mg of EM and 300 mg of TDF. The same label claim tablets were considered for method validation purpose. Validations parameters covered for System suitability evaluation, Specificity, Precision (method precision and intermediate precision), Linearity, Accuracy/Recovery, stability of solutions, suitability of Filter papers and Robustness parameters as per ICH recommendation for “Validation of Analytical Procedures: Text and Methodology - Q2 (R1)”.
System suitability evaluation
Standard solution was prepared and injected for five replicate injections and observed for peak area of EL, CO, EM and TDF. Theoretical plate count, tailing factor, Resolution and %RSD is evaluated.
Specificity
Equal proportions of excipients are mixed as per QUAD formula. This placebo powder which is equivalent to individual tablet weight is transferred to dissolution vessel which contains dissolution medium. Rotation of the paddle is maintained at 100 rpm for 60 min. Placebo solution is withdrawn from dissolution vessel and filtered through 0.22 μ PVDF filter paper and analyzed in UPLC system.
Precision
Stribild tablets were tested for precision of the method for intra and inter day precision for six individual preparations. All test samples were analyzed after subjecting it in dissolution vessels as per proposed time intervals. Measured % dissolution at every time interval and % RSD is determined for same values for every time point.
Linearity
Linearity study was assessed by preparing the test solutions ranging from 10%-120% level using concentrated standard stock solutions for each drug. Linearity curves were constructed, by plotting the concentration (μg/ml) against peak area for each drug. The calculation of regression line was employed by the method of least squares.
Accuracy
Known amounts of EL, CO, EM and TDF reference substances were transferred to dissolution bowls at 10%, 80%, 100% and 120% levels along with tablets placebo. Dissolution was run for the samples asper OGD recommended dissolution test conditions. Triplicate preparations are made at each level.
Solution stability
Standard and sample solutions were periodically injected and verified the response of the peaks from the solutions stored at bench top condition (25°C) or cooler temperature (2-8°C). The chromatograms obtained by the RP-UPLC method were evaluated for area. Tests results of area counts are compared against freshly prepared solutions of standard and sample solutions.
Filter evaluation
To demonstrate the filter paper interference, standard and sample solutions were filtered using 0.22 μ PVDF and Nylon membrane filters by initially discarding 2-3 ml of aliquots from the filters. The filters were pre saturated with dissolution medium prior to filtration. Results are compared against centrifuged sample areas.
Robustness
Robustness study was assessed by making deliberate changes in the optimized chromatographic conditions and impact was noted for USP plate count, Tailing factor and resolution between each drug. Accordingly conditions were modified for flow rate of 0.3 ml (± 10%), wavelength of 260 nm (± 5 nm), temperature of 45°C (± 5°C) and Organic ratio in gradient elution (± 2% absolute). For each robustness experiment, one parameter was modified and remaining chromatographic conditions were kept as such.
Conclusion
Method was validated as per ICH general requirement for Dissolution test procedure. The result obtained from specificity experiment is showing that there is no placebo interference observed at the retention times of EL, CO, EM and TDF. Detection wavelength of 260 nm is found to specific and could able to provide precise area counts for each drug. Calibration curves depicting a proper linearity response from concentration versus area observed for each drug with correlation coefficients greater than 0.99. Recovery/Accuracy results confirming that satisfactory drug recovery is seen on proposed test concentrations for each drug. The developed and validated method could able to quantify the drugs of EL, CO, EM and TDF in tablet formulation for dissolution profiling at precise and accurate levels at very short run time. Hence developed method could be useful to pharmaceutical analytical laboratories to release the profiling results at faster rate.
Acknowledgment
The author wish to thank the management of Aurobindo Pharma limited (A Division of APL Research centre, Bachupally, Hyderabad, India) for supporting this work.
References
- L.O. Jacqueline, M.S. Linda, K. Olga, Annals of Pharmacother., 2012, 46(12), 1671-1677.
- C.M. Perry, Drugs, 2014, 74(1), 75-97.
- J.R. Arribas, G. Pialoux, J. Gathe, G.D. Perri, J. Reynes, P. Tebas, T. Nguyen, R. Ebrahimi, K. White, D. Piontkowsky, Lancet Infect. Dis., 2014, 14(7), 581-589.
- V.V. Babu, R. Sharma, K. Pankaj, I. Singhvi, Asian J. Chem., 2014, 26(18), 6233-6237.
- R. Chinnalalaiah, P.R. Kumar, A. Srinivasa Rao, J. Chromatogra. Sci., 2016.
- N. Mallikarjunarao, D. GowriSankar, Indian J. Pharmaceut. Edu. Res., 2016, 50(1), 205-211.
- J.R. Ram, V.K. Kumar, N.A. Raju, Pharmaceutical Methods, 2014, 5(1), 7-13.
- P.P. Rao, D.M. Reddy, D. Ramachandran, World J. Pharm. Sci., 2014, 2(12), 1822-1829.
- V.V. Raveendra Babu, K.P. Sharma, I. Singhvi, Asian J. Chem., 2014, 26(18), 6233-6237.
- N. Khaleel, Sk. Abdul Rahaman, Int. J. Pharm., 2015, 5(3), 991-1002.
- P.R. NagaLakshmi, P. Prahlad, S.K. Mastanamma, N. Ravindra, M.V. Basaveswararao, Int. J. Pharm. Pharmaceut. Sci., 2016, 8(4), 1-8.
- Validation of Analytical Procedures: Text and Methodology, ICH, 2005, Q2 (R1).
- Official Methods of Analysis, Association of Official Analytical Chemists International, Arlington, 15th (Edn.), VA, XVII, 1990.
- US FDA Guidance, Analytical Procedures and Methods Validation, 2000.
- Validation of Compendial Methods <1225>, “The United States Pharmacopeia, 2016.
- Guideline for submitting samples and Analytical Data for Methods Validation, US Food and Drug Administration, 1987.
- M.E. Swartz, I.S. Krull, Pharmaceutical Technology, 2006.
- S.W. Baertschi, K. Alsante, R.A. Reed, Pharmaceutical stress testing: Predicting drug degradation, Informa Healthcare, 2005.
- D.M. Bliesner, Validating chromatographic methods: A Practical Guide, Wiley, 2006.