Research Article - Der Pharma Chemica ( 2020) Volume 12, Issue 4
Visible Light Photoredox-Catalyzed Synthesis of Quinazolinone Derivatives and their cytotoxicity
Jeshma Kovvuri*Jeshma Kovvuri, Vardhaman College of Engineering (Autonomous), Hyderabad, Telangana, 501218, India, Email: dr.jeshmakovvuri@gmail.com
Received: 21-Oct-2019 Accepted Date: Jul 08, 2020 ; Published: 28-Aug-2020
Abstract
Visible light promoted efficient and eco-friendly photocatalytic method for the synthesis of quinazolinones. This protocol involves commencing readily available substituted isatins and 2-aminobenzamide by using Rose Bengal as an efficient recyclable photocatalyst. This method is operationally simpler and selective, carried out in shorter reaction time with visible light in higher yields. Using this protocol, a series of twenty compounds has been synthesized, all the synthesized compounds were evaluated for their cytotoxic potential on three human cancer cell lines and most of the compounds exhibited moderate to good cytotoxic activity, while some of them showed promising cytotoxicity with IC50 values ranging between 1.13 μM-1.77 μM.
Keywords
Cytotoxicity, Quinazolinones, Rose Bengal, Photocatalyst, Visible light
Introduction
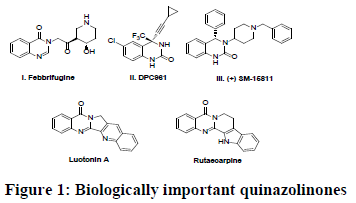
Quinazolinone derivatives are important bioactive nitrogen-containing heterocycles and are present in several natural products such as vitamins, alkaloids, etc. Due to their diverse pharmacological and biological activities [1] quinazolinone scaffolds are very important in the synthesis of various biologically active compounds. Recently, these compounds are reported to exhibit as gene associated peptide and vasopressin receptor antagonists [2,3]. Febrifugine (I), a quinazolinone alkaloid was first isolated from the Chinese herb Dichoria febrifuges. It has important biological properties such as antimalarial [4], anticancer [5]and anti-inflammatory [6,7]. DPC-961 (II) and SM-15811 (III) (Figure 1) are compounds having quinazolinone ring and are known to exhibit 2nd generation anti-HIV activity, ion exchanger properties and also used to treat heart diseases and luotonine A showed excellent cytotoxicity towards the murine leukemia and rutaecarpine used extensively as a remedy for headache, cholera, and dysentery. Considering the significance of quinazolinones from an application perspective, although a number of methods have been made to construct the quinazolinones [8], these routes mainly rely on using anthranilic acid or its derivatives as starting materials. Generally, metal catalysed reductive cyclization of anthranilamide with ketones, aldehydes and isatins by the alternative approaches used for the synthesis of substituted quinazolinones [9].
However, these methods have some eco-friendly aspects but these methods are associated with several drawbacks such as usage of hazardous organic solvents, reagents, metal catalysts, harsh reaction conditions and low yields. So, there is a need for the development of green, sustainable, efficient and safety and alternative method for the synthesis of quinazolinones via visible light promoted metal free photocatalyst in environmental aspect was highly desirable.
In the present scenario, visible light photo redox catalysis has fascinated the chemists in the forefront of the organic synthesis due to its attractive features like mild reaction conditions, safe, cheap, abundant and renewable energy source [10]. It is a dominant tool to complete novel organic chemical transformations through single-electron-transfer pathway. Continuing our efforts towards the synthesis of heterocyclic scaffolds [11] herein, we developed a visible light driven environmentally benign process for the synthesis of quinazolinones from readily available 2-aminobenzamide and isatins at room temperature under air by using organic dye such as Rose Bengal as a recyclable photocatalyst.
Materials and Methods
All chemicals, reagents were purchased from the commercial sources and were used without further purification. Reactions were monitored by TLC on silica gel glass plate containing 60 GF-254, and visualization was done by UV light and iodine vapour. 1H and 13C NMR spectra were recorded on Bruker UXNMR/XWIN-NMR (300 MHz) or Innova Varian-VXR-unity (400 MHz, 500 MHz) instruments. Chemical shifts were expressed in parts per million (in ppm) downfield from TMS expressed as internal standard and coupling constants are expressed in Hz. 1H NMR spectral data were reported in the following order: multiplicity (s: singlet; brs: broad singlet; d: doublet; dd: doublet of doublets; t: triplet; m: multiplet), coupling constants in Hz, and number of protons. ESI mass spectra were recorded on a Micromass Quattro LC using ESI+ software with capillary voltage 3.98 kV and an ESI mode positive ion trap detector. High resolution mass spectra were recorded on a QSTAR XL Hybrid MS-MS mass spectrometer. Melting points were determined with an electro thermal digital melting point apparatus IA9100 and are uncorrected.
General reaction procedure for the preparation of compounds (3a-3t)
All the reactions were performed using the following procedure.
To the solution of 2-aminobenzamide (1) (1 mmol) in ethanol add substituted isatin (2) (1 mmol) and Rose Bengal (2 mol%), under the visible light (15 W) setup at room temperature for 2 h, appearance of precipitate indicates the product formation and which was also monitored by the TLC. The solvent was removed by the rota vacuum then purified by the column chromatography to offered quinazolinones in good to excellent yields.
1H-spiro[indoline-3,2’-quinazoline]-2,4’(3’H)-dione (3a)
Dirty white solid; 87% yield; mp 290°C-295°C; 1H NMR (300 MHz, DMSO-d6): δ 9.76 (s, 1H), 7.80 (d, J=7.9 Hz, 1H), 7.52 (d, J=7.4 Hz, 1H), 7.29-7.21 (m, 2H), 7.03 (t, J=7.4 Hz, 2H), 6.85 (d, J=8.2 Hz, 1H), 6.76 (t, J=7.6 Hz, 1H), 6.67 (d, J=8.1 Hz, 1H), 6.15 (bs, 1H); 13C NMR (75 MHz, DMSO-d6): δ 175.79, 164.14, 146.53, 141.88, 132.98, 130.38, 129.18, 126.73, 124.94, 121.93117.05, 114.07, 113.77, 109.96, 70.84; IR (KBr pellets) υ: 3363, 3339, 3179, 3050, 1730, 1706, 1663, 1620, 1509, 1483, 1470, 1359, 1324, 1185, 748 cm-1; ESI-MS : m/z=266 (M+H) +; HRMS (ESI) m/z for C15H12O2N3 calculated m/z: 266.0924, found m/z: 266.0922 (M+H) +.
5-chloro-1H-spiro[indoline-3,2’-quinazoline]-2,4’(3’H)-dione (3b)
White solid; 80% yield; mp 289°C-294°C; 1H NMR (300 MHz, DMSO-d6): δ 9.98 (s, 1H), 7.76 (t, J=7.6 Hz, 2H), 7.23 (s, 2H), 6.96 (t, J=7.2 Hz, 1H), 6.82-6.58 (m, 4H) ; 13C NMR (75 MHz, DMSO-d6): 13C NMR (75 MHz, DMSO-d6): δ 175.80, 164.10, 145.72, 141.31, 139.09, 133.55, 133.28, 130.99, 127.05, 117.82, 113.84, 112.42, 83.96, 70.76; IR (KBr pellets) υ: 3252, 1731, 1660, 1651, 1613, 1514, 1504, 1483, 1440, 1359, 1268, 1190, 753, 694 cm-1; ESI-MS: m/z=300 (M+H)+; HRMS (ESI) m/z for C15H11O2N3Cl calculated m/z: 300.0534, found m/z: 300.0534 (M+H)+.
5-bromo-1H-spiro[indoline-3,2’-quinazoline]-2,4’(3’H)-dione (3c)
Light red solid; 77% yield; mp 280°C-285°C; 1H NMR (300 MHz, DMSO-d6): δ 10.02 (s, 1H), 7.79 (d, J=7.55 Hz, 1H), 7.61 (d, J=1.70 Hz, 1H), 7.48-7.51 (m, 1H), 7.40 (dd, J=1.88 & 8.30 Hz, 1H), 7.24 (dt, J=1.51 & 8.30 Hz, 1H), 6.74-6.78 (m, 2H), 6.68 (d, J=7.93 Hz, 1H), 6.43 (s, 1H); 13C NMR (75 MHz, DMSO-d6): δ 175.28, 163.92, 146.04, 140.91, 133.01, 132.89, 131.17, 127.81, 126.74, 117.32, 113.88, 113.74, 111.67, 70.83; IR (KBr pellets) υ:1731, 1660, 1614, 1513, 1482, 1434, 1358, 1302, 1268, 1189, 1145, 1122, 817, 752, 628, 566, 538 cm-1; ESI-MS: m/z=343 (M)+; HRMS (ESI) m/z for C15H11O2N3 Br calculated m/z: 343.0029, found m/z: 343.0030 (M)+.
5-flouoro-1H-spiro[indoline-3,2’-quinazoline]-2,4’(3’H)-dione (3d)
White solid; 79% yield; mp 285-287°C; 1H NMR (300 MHz, DMSO-d6): δ 9.98 (s, 1H), 7.78 (m, 2H), 7.23 (bs, 2H), 7.01 (t, J =7.36 Hz, 1H), 6.81-6.66 (m, 3H), 6.58 (s, 1H); 13C NMR (75 MHz, DMSO-d6): δ 175.80, 164.10, 146.14, 137.63, 133.09, 130.43, 126.80, 117.40, 116.80, 113.94, 113.83, 112.67, 110.70, 71.12; IR (KBr pellets) υ: 3282, 1741, 1661, 1632, 1613, 1520, 1185, 1146, 1124, 1042, 817, 758, 710, 693, 636, 616 cm-1; ESI-MS: m/z=284 (M+H)+; HRMS (ESI) m/z for C15H11O2N3F calculated m/z: 284.0829, found m/z: 284.0831 (M+H)+.
5-iodo-1H-spiro[indoline-3,2’-quinazoline]-2,4’(3’H)-dione (3e)
White solid; 80%yield; mp 240-245°C; 1HNMR(300MHz, DMSO-d6): 10.04 (s, 1H), 7.77 (d, J=8.6 Hz, 3H), 7.58 (d, J=7.9 Hz, 1H), 7.25 (t, J=7.55 Hz, 1H), 6.76-6.64 (m, 4H); 13C NMR (75 MHz, DMSO-d6): δ 175.02, 164.18, 145.72, 141.31, 139.09, 133.55, 133.28, 130.99, 127.05, 117.82, 113.84, 112.42, 83.96, 70.76; IR (KBr pellets) υ: 3282, 1741, 1661, 1613, 1520, 1484, 1425, 1371, 1335, 1299, 1265, 1185, 1146, 1124, 1042, 817, 758, 636, 616 cm-1; ESI-MS: m/z=390 (M)+; HRMS (ESI) m/z for C15H11O2N3I calculated m/z: 391.9890, found m/z: 391.9892 (M)+.
5-methyl-1H-spiro[indoline-3,2-quinazoline]-2,4’(3’H)-dione (3f)
White solid; 85% yield; mp 150-155°C; 1H NMR (300 MHz, DMSO-d6): δ 9.81 (s, 1H), 7.75 (d, J=7.17 Hz, 1H), 7.40 (brs, 1H), 7.34 (s, 1H), 7.22 (t, J=8.30 Hz, 1H), 7.07 (d, J=7.55 Hz, 1H), 6.65-6.80 (m, 3H), 6.49 (s, 1H), 2.30 (s, 3H); 13C NMR (75 MHz, DMSO-d6 ): δ 175.77, 164.19, 146.40, 139.20, 132.94, 131.16, 130.62, 129.00, 126.75, 125.49, 117.06, 113.97, 113.72, 109.73, 70.92, 20.48; IR (KBr pellets) υ: 3314, 2922, 2853, 1706, 1655, 1613, 1513, 1489, 1357, 1329, 1270, 1207, 1154, 811, 751, 698 cm-1; ESI-MS: m/z=280 (M+H); HRMS (ESI) m/z for C16H14O2N3 calculated m/z: 280.1080, found m/z: 280.1081 (M+H) +.
5-nitro-1H-spiro[indoline-3,2’-quinazoline]-2,4’(3’H)-dione (3g)
White solid; 84% yield; mp 230-235°C; 1H NMR (300 MHz, DMSO-d6): δ 10.85 (s, 1H), 8.19-8.40 (m, 3H), 7.71 (d, J=7.17 Hz, 1H), 7.14 (s, 1H), 7.24 (t, J=8.49 Hz, 1H), 7.00 (d, J=8.68 Hz, 1H), 6.75 (t, J=7.55 Hz, 1H), 6.67 (d, J=7.93 Hz, 1H); 13C NMR (75 MHz, DMSO-d6): δ 176.04, 163.82, 148.22, 145.90, 142.41, 133.19, 130.02, 127.10, 126.81, 120.62, 117.62, 113.90, 109.99, 70.56; IR (KBr pellets) υ: 3342, 3021, 2860, 1727, 1649, 1616, 1522, 1482, 1405, 1339, 1296, 1252, 1229, 1189, 1143, 1121, 1085, 837, 746, 733, 690, 635, 550 cm-1; ESI-MS: m/z=311 (M+H)+; HRMS (ESI) m/z for C15H11O4N4 calculated m/z: 311.0774, found m/z: 311.0775 (M+H)+.
1-benzyl-5-chloro-1-H-spiro[indoline-3,2-quinazoline]-2,4(3H)-dione (3h)
White solid; 79% yield; mp 150°C-155°C; 1H NMR (DMSO-d6, 300 MHz) δ: 8.1 (s, 1H), 7.8 (d, J=7.9 Hz, 1H), 7.6 (s, 1H), 7.55 (t, J=8.1 Hz, 2H), 6.9 (s, 1H ), 6.77-6.68 (m, 4H), 6.5 (bs, 1H), 6.0 (bs, 1H), 4.9 (s, 1H), 4.8 (s, 2H, ), 3.8 (d, 1H); 13C NMR (75 MHz, DMSO-d6): δ 145.16, 141.26, 133.8, 133.4, 130.75, 129.4, 126.09, 123.8, 121.6, 116.4, 115.41, 113.70, 107.65, 69.27, 41.89; IR (KBr pellets, υ: 3758, 3713, 3707, 3685, 3644, 3625, 3583, 3270, 2923, 2357, 1737, 1731, 1715, 1666, 1660, 1650, 1644, 1633, 1613, 1514, 1504, 1494, 1485 1434, 1336 cm-1; ESI-MS m/z: 389 (M+); HRMS (ESI) m/z for C22H17ClN3O2 calculated m/z: 390.1003, found m/z: 390.1004 (M+H)+.
5-methoxy-1H-spiro[indoline-3,2’-quinazoline]-2,4’(3’H)-dione (3i)
White solid; 85% yield; mp 270°C-275°C; 1H NMR (300 MHz, DMSO-d6): δ 9.74 (s, 1H), 7.79 (d, J=7.8 Hz, 1H), 7.5 (s, 1H), 7.45 (bs, 1H), 7.1 (s, 1H ), 6.81-6.45 (m, 4H ), 6.45 (s, 1H), 3.75 (s, 3H); 13C NMR (75 MHz, DMSO-d6): δ 175.8, 163.94, 155.10, 154.46, 146.58, 134.95, 132.87, 130.34, 128.28, 127.81, 126.64, 123.55, 116.93, 116.58, 115.37, 144.08, 113.69, 111.36, 110.39, 108.35, 71.18, 55.29; IR (KBr pellets) υ: 3314, 3215, 2924, 1719, 1648, 1609, 1513, 1485, 1440, 1357, 1296, 1270, 1234, 1162, 1043, 1024, 824, 794, 756, 715, 698, 672, 621, 578 cm-1; ESI-MS : m/z=296 (M+H) +; HRMS (ESI) m/z for C16H14O3N3 calculated m/z : 296.1029, found m/z: 296.1030 (M+H) +.
5,6-dimethyl-1H-spiro[indoline-3,2’-quinazoline]-2,4’(3’H)-dione (3j)
White solid; 87% yield; mp 320°C-325°C; 1H NMR (300 MHz, DMSO-d6): δ 9.81 (s, 1H), 7.75 (d, J=7.2 Hz, 1H), 7.40 (bs, 1H), 7.34 (s, 1H), 7.22 (t, J=8.30 1H), 7.07 (d, J=7.55 Hz, 1H), 6.80-6.65 (m, 2H), 6.49 (s, 1H), 2.39 (s, 6H); 13C NMR (75 MHz, DMSO-d6 ): δ 175.78, 164.20, 146.41, 139.20, 132.94, 131.16, 130.62, 129.00, 126.75, 125.49, 117.06, 113.97, 113.72, 109.73, 70.92, 20.48; IR (KBr pellets) υ: 3311, 3301, 2902, 2833, 1706, 1685, 1623, 1513, 1489, 1357, 1329, 1270, 1207, 1154, 811, 751, 698 cm-1; ESI-MS : m/z=293 (M+H) +; HRMS (ESI) m/z for C17H16O2N3 calculated m/z: 294.1237, found m/z: 294.1239 (M+H) +.
1-benzyl-1'H-spiro[indoline-3,2'-quinazoline]-2,4'(3'H)-dione (3k)
White solid; 82% yield; mp 240°C-245°C; 1H NMR (300 MHz, DMSO-d6): δ 8.04 (s, 1H), 7.79 (d, J=7.4 Hz, 1H), 7.68 (s, 1H), 7.59 (d, J=8.2 Hz, 1H), 7.12 (t, J=7.2 Hz, 1H), 6.93-6.84 (m, 3H ), 6.77-6.68 (m, 5H), 5.94 (s, 2H ), 4.72 (s, 2H); 13C NMR (75 MHz, DMSO-d6 ): δ 174.05, 171.39.164.31, 149.33, 146.18, 142.32, 134.91, 131.78, 130.43, 128.21, 127.11, 126.86, 124.83, 120.30, 117.44, 114.89, 113.88, 109.01, 107.78, 100.53, 70.65, 42.92; IR (KBr pellets) υ: 3329, 3179, 3054, 2918, 1705, 1667, 1615, 1511, 1489, 1466, 1454, 1362, 1314, 1264, 1175, 1142, 993, 749, 696, 678, 630 cm-1; ESI-MS : m/z=356 (M+H) +; HRMS (ESI) m/z for C22H18O2N3 calculated m/z: 356.1393, found m/z: 356.1395 (M+H) +.
1-(benzo[d][1,3]dioxol-5-ylmethyl)-1H-spiro[indoline-3,2-quinazoline]2,4(3H)-dione (3l)
White solid; 84% yield; mp 260°C-265°C; 1H NMR (300 MHz, DMSO-d6): δ 8.01 (s, 1H), 7.79 (d, J=7.6 Hz, 1H), 7.66 (s, 2H ), 7.57 (d, J=7.2 Hz, 1H), 7.48 (d, J=7.3 Hz, 1H), 7.31-6.68 (m, 7H), 5.94 (s, 2H), 4.72 (s, 2H); 13C NMR (75 MHz, DMSO-d6): δ 174.05, 164.22, 147.44, 146.47, 146.28, 142.27, 133.03, 130.38, 128.64, 126.79, 124.75, 122.64, 120.31, 117.31, 114.05, 113.84, 109.16, 107.80, 107.41, 100.57, 70.67, 42.67; IR (KBr pellets) υ: 3193, 3059, 1658, 1670, 1615, 1487, 1467, 1444, 1356, 1272, 1244, 1190, 1174, 1143, 1100, 1036, 743, 695 cm-1; ESI-MS: m/z=400 (M+H)+; HRMS (ESI) m/z for C23H18O4N3 calculated m/z: 400.1291, found m/z: 400.1293 (M+H)+.
1-(benzo[d][1,3]dioxol-5-ylmethyl)-5-chloro-1H-spiro[indoline-3,2-quinazoline]2,4(3H)-dione (3m)
White solid; 79% yield; mp 195°C-200°C; 1H NMR (300 MHz, DMSO-d6): δ 8.11 (d, J=7.17 Hz, 1H), 7.68 ( d, J=9.63 Hz, 1H ), 7.52 (t, J=7.93 Hz, 1H ), 7.41-7.25 ( m, 3H ), 6.99 (t, J=6.42 Hz, 2H), 6.85-674 (m, 4H), 6.50 (s, 1H), 5.92 (d, J=7.36, 2H), 4.81 (s, 1H); 13C NMR (75 MHz, DMSO-d6): δ 174.57, 166.34, 145.08, 136.68, 133.49, 131.48, 131.20, 130.37, 127.83, 125.66, 124.99, 123.36, 120.15, 116.69, 116.12, 114.68, 111.76, 111.06, 107.59, 107.05, 100.35, 42.59; IR (KBr pellets) υ: 3272, 1670, 1659, 1651, 1647, 1632, 1614, 1573, 1562, 1551, 1536, 1532, 1513, 1492, 1483, 1470, 1453, 1432, 1415, 1391, 1370, 1335, 1171, 1252, 1123, 1080, 1028, 817, 753 cm-1; ESI-MS: m/z=434 (M+H)+; HRMS (ESI) m/z for C23H17O4N3Cl calculated m/z: 434.0903, found m/z: 434.0905 (M+H)+.
1-(benzo[d][1,3]dioxol-5-ylmethyl)-5-bromo-1H-spiro[indoline-3,2-quinazoline]2,4 (3H)-dione (3n)
White solid; 76% yield; mp 160°C-165°C; 1H NMR (DMSO-d6, 300 MHz, ppm) δ: 7.53, (t, J=8.3 Hz, 3H), 7.3 (bs, 1H), 7.20 (t, J=8.3 Hz, 3H), 6.7 ( d, J=7.9 Hz, 3H), 6.62 ( t, J=7.7 Hz, 2H), 5.9 (bs, 4H); 13C NMR (75 MHz, DMSO-d6): δ 171.47, 164.31, 149.33, 146.18, 142.32, 134.91, 133.07, 131.78, 128.26, 127.11, 126.86, 124.83, 122.67, 120.30, 117.44, 116.44, 114.89, 113.88, 133.64, 109.01, 70.65, 42.92; IR (KBr pellets) υ: 3411, 2923, 2853, 1731, 1650, 1585, 1546, 1487, 1452, 1402, 1315, 1257, 1151, 743 cm-1; ESI-MS m/z: 433 (M+). HRMS (ESI) m/z for C22H17BrO2N3 calculated m/z: 434.0498, found m/z: 434.0499 (M+H)+.
7-fluoro-1H-spiro[indoline-3,2’-quinazoline]-2,4’(3’H)-dione (3o)
light white solid; 79% yield; mp 310°C-315°C; 1H NMR (300 MHz, DMSO-d6): δ 9.96 (s,1H), 7.78 (d, J=7.5 Hz, 2H), 7.70 (s, 1H), 7.23 (s, 1H) 7.09, 6.99 (t, J=7.36 Hz, 1H), 6.81-6.78 (m, 2H), 6.68 (s, J=7.9 Hz, 1H) 6.58 (s, 1H); 13C NMR (75 MHz, DMSO-d6): δ 175.80, 164.10, 146.14, 137.63, 133.09, 130.43, 126.80, 117.40, 116.80, 113.94, 113.83, 112.67, 110.70, 71.12; IR (KBr pellets) υ: 3326, 3177, 1732, 1614, 1588, 1516, 1486, 1370, 1358, 1314, 1272, 1254, 1203, 1152, 1083, 1033, 902, 736, 686, 661, 603, 578, 527 cm-1; ESI-MS: m/z=284 (M+H)+; HRMS (ESI) m/z for C15H11O2N3F calculated m/z: 284.0829, found m/z: 284.0831 (M+H)+.
1’H-spiro[cyclohexane-1,2’-quinazoline]-4’(3’H)-one (3p)
White solid; 86% yield; mp 235°C-239°C; 1H NMR (300 MHz, DMSO-d6) δ 7.80 (s, 1H), 7.25, (s, 1H), 6.85 (d, J=7.93 Hz, 2H ), 5.24 (s, 1H), 2.77 (bs, 1H), 2.17 (s, 2H), 1.82-1.45 (m, 8H); 13C NMR (75 MHz, DMSO-d6): δ 163.69, 145.95, 132.90, 127.12, 116.85, 114.25, 67.67, 36.95, 24.17, 21.00; IR(KBr pellets) υ: 3365, 3169, 3023, 2926, 2852, 1647, 1504, 1482, 1416, 1381, 1323, 1268, 1143, 756 cm-1; ESI-MS: m/z=217 (M+H)+; HRMS (ESI) m/z for C13H17ON2 calculated m/z: 217.1335, found m/z: 217.1336 (M+H)+.
6-chloro-1'H-spiro[indoline-3,2'-quinazoline]-2,4'(3'H)-dione (3q)
White solid; 81% yield; mp 284°C-289°C; 1H NMR (300 MHz, DMSO-d6): δ 8.79 (s, 1H), 6.51 (d, J=7.82 Hz, 3H), 6.33 (d, J=7.93 Hz, 1H), 5.97 (t, J=8.23 Hz, 1H), 5.51-5.39 (m, 4H); 13C NMR (75 MHz, DMSO-d6): δ 174.46, 163.62, 145.16, 140.78, 138.53, 132.72, 130.42, 126.49, 117.26, 111.86, 83.40, 70.20, 59.20, 19.43, 13.11; IR (KBr pellets) υ: 3256, 1734, 1661, 1651, 1612, 1516, 1505, 1483, 1442, 1358, 1267, 1191, 754, 695 cm-1; ESI-MS: m/z=300 (M+H)+; HRMS (ESI) m/z for C15H11O2N3Cl calculated m/z: 300.0532, found m/z: 300.0534 (M+H)+.
6-bromo-1'H-spiro[indoline-3,2'-quinazoline]-2,4'(3'H)-dione (3r)
Brown solid; 73% yield; mp 275°C-280°C; 1H NMR (300 MHz, DMSO-d6): δ 8.77 (s, 1H), 6.47 (t, J=8.68 Hz, 3H), 6.30 (t, J=7.93 Hz, 1H), 5.95 (t, J=7.55 Hz, 1H), 5.49-5.37 (m, 4H); 13C NMR (75 MHz, DMSO-d6): δ 175.85, 165.02, 146.56, 142.15, 139.92, 134.38, 131.82, 127.88, 118.68, 114.67, 113.25, 84.79, 71.59, 60.59, 14.50; IR (KBr pellets) υ: 1735, 1661, 1617, 1514, 1483, 1435, 1358, 1301, 1267, 1187, 1146, 1121, 816, 754, 628, 564, 539 cm-1; ESI-MS: m/z=343 (M)+; HRMS (ESI) m/z for C15H11O2N3Br calculated m/z: 344.0029, found m/z: 343.0031 (M)+.
4-methyl-1'H-spiro[indoline-3,2'-quinazoline]-2,4'(3'H)-dione (3s)
White solid; 76% yield; mp 152-154°C; 1H NMR (300 MHz, DMSO-d6): δ 9.98 (s, 2H), 7.57 (t, J=7.55 Hz, 5H), 7.56 (s, 1H), 6.99 (s, 2H), 2.03 (d, J=7.36 Hz, 3H); 13C NMR (75 MHz, DMSO-d6 ): δ 175.24, 163.54, 155.97, 145.59, 137.08, 132.54, 126.25, 116.85, 116.25, 115.94, 113.39, 112.11, 111.79, 110.15, 70.57; IR (KBr pellets) υ: 3317, 2921, 2851, 1708, 1653, 1613, 1514, 1486, 1357, 1328, 1270, 1205, 1151, 817, 755, 698 cm-1; ESI-MS: m/z=280 (M+H); HRMS (ESI) m/z for C16H14O2N3 calculated m/z: 280.1081, found m/z: 280.1083 (M+H) +.
1-(benzo[d][1,3]dioxol-5-ylmethyl)-6-chloro-1'H-spiro[indoline-3,2'-quinazoline]-2,4'(3'H)-dione (3t)
White solid; 79% yield; mp 195°C-200°C; 1H NMR (300 MHz, DMSO-d6): δ 8.18 (d, J=7.18 Hz, 1H), 7.78 (s, 1H), 7.61(s, 1H), 7.50-7.35 (m, 3H), 7.11-7.06 (m, 1H), 76.93-6.83 (m, 4H), 6.60 (s, 1H), 6.02 (d, J=7.17 Hz, 2H), 4.96 (s, 2H); 13C NMR (75 MHz, DMSO-d6): δ 166.06, 152.48, 147.05, 144.80, 136.40, 133.22, 130.09, 127.55, 124.72, 119.87, 116.41, 114.40, 110.78, 106.77, 100.07, 42.65; IR (KBr pellets) υ: 3272, 1670, 1659, 1651, 1647, 1632, 1614, 1573, 1562, 1551, 1536, 1532, 1513, 1492, 1483, 1470, 1453, 1432, 1415, 1391, 1370, 1335, 1171, 1252, 1123, 1080, 1028, 817, 753 cm-1; ESI-MS: m/z=434 (M+H)+; HRMS (ESI) m/z for C23H17O4N3Cl calculated m/z: 434.0903, found m/z: 434.0905 (M+H)+.
MTT Assay
The cytotoxicity of these compounds was determined using MTT assay.13 Cancer cells (DU-145, MCF-7, HeLa and A549) were used in this assay. 1×104 cells/well were seeded in 200 μl Dulbecco’s modified Eagle’s medium (DMEM), supplemented with 10% FBS in each well of 96-well micro culture plates and incubated for 24 h at 37°C in a CO2 incubator. All the derivatives diluted to the desired concentrations (500 nM, 1 μM, 5 μM, 10 μM, 25 μM, 50 μM, 75 μM, 100 μM and 150 μM) in culture medium, were added to the wells with respective vehicle control. Doxorubicin treated cells, in the same concentration range were used as standards. After 48 h of incubation, 10 μl MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromide) (5 mg/ml) was added to each well and the plates were further incubated for 4 h. Then the supernatant from each well was carefully removed, Formosan crystals were dissolved in 100 μl of DMSO and absorbance at 570 nm wavelength was recorded at a wavelength of 540 nm using an ELx800 micro plate reader (BioTek, USA) [12,13].
Conclusion
In conclusion, a simple, efficient and environmentally benign visible light mediated method for the synthesis of quinazolinone derivatives has been developed by employing Rose Bengal as a recyclable metal free photocatalyst in ethanol. The advantages of this method include its simplicity in operation, use of renewable visible light as an energy source, eco-friendly reactions, higher yields and absence of side products. Using this protocol, a series of twenty compounds has been synthesized and this is a practical and economical method for the development of functionalized quinazolinones. The synthesized compounds were evaluated for their cytotoxic potential on selected human cancer cell lines. Some of these compounds showed IC50 values ranging between 1.13 μM to 1.77 μM against DU-145 cell line.
Acknowledgements
J.K thanks to Vardhaman College of Engineering for providing support.
References
[1] A.A.A. Abdel-Aziz, A. Laila, A. Zeid et al., Bio. Med. Chem., 2016, 24: p. 3818.
[2] A.Al-Obaid, S. Abdel-Hamide, H. El-Kashef et al., Eur. J. Med. Chem., 2009, 44: p. 2379.
[3] F. A. Al-Omary, L. A. Abou-Zeid, M. N. Nagi et al., Bio. Med. Chem., 2010, 18: p. 2849.
[4] R. Lin, S. G. Johnson, P. J. Connolly et al., Bio. Med. Chem. Lett., 2009, 19: p. 2333.
[5] H. T. Fahmy, S. G. Rostom, M. N. Saudi et al., Arch Pharm., 2003, 336: p. 216.
[6] M. Y. Jang, S. S. K. Jonghe, J. Anne, P. Herdewijn, Bio. Med. Chem., 2011, 19: p. 702.
[7] A. A. Bekhit, H. T. Fahmy, S. A. Rostom et al., Eur. J. Med. Chem., 2003, 38: p. 27.
[8] A. Shaabani, A. Maleki, H. Mofakham et al., J. Chem. Sci., 2016, 128: p. 657.
[9] B. V. Subba Reddy, A. Venkateswarlu, Ch. Madan et al., Tet. Letters., 2011, 52: p. 1891.
[10] J. M. R. Narayanam and C. R. J. Stephenson, Chem. Soc. Rev., 2011, 40, 102
[11] R. Maurya, P. R. Adiyala, D. Chandrasekhar et al., Sci., 2014, 16: p. 466
[12] J. Hu, J. Wang, T. H. Nguyen et al., J. Org. Chem., 2013, 9: p. 1977.
[13] T. Mosmann, J. Immunol. Methods 1983, 65: p. 55.