Research Article - Der Pharma Chemica ( 2017) Volume 9, Issue 2
Zn (II) and Cd(II) Complexes of 3-(2-(2-hydroxybenzylidene)hydrazinyl) quinoxalin-2(1H)-one: Synthesis, Characterization and DNA Binding Studies
Sukanya P1 and Venkata Ramana Reddy CH22Department of Chemistry, Jawaharlal Nehru Technological University, Hyderabad 500085, India
Abstract
Zn (II) and Cd(II) complexes of the tridentate Schiff base ligand 3-(2-(2-hydroxybenzylidene)hydrazinyl) quinoxalin-2(1H)-one(L) have been synthesized and characterized by 1H-NMR,IR, UV-Visible, Mass spectroscopy and CHN analysis data. The ligand (L) formed 1:2 complexes, ML2 with M=Zn(II) and Cd(II) and behaved as O,N,O-tridentate ligand. The complexes were found to be fairly stable. The interaction of the complexes with Calf Thymus DNA was investigated by UV spectroscopic studies and the intrinsic binding constant, Kb values are calculated. The base binding constants of the DNA with Zn(II) and Cd(II) complexes were found to be 7.18 × 105 and 8.18 × 109 M-1 respectively.
Keywords
Tridentate ligand, Schiff base, Octahedral geometry, Calf thymus DNA
Introduction
Quinoxalins belongs to an important class of Nitrogen containing heterocyclic compounds known as Diazonaphthalenes. They process biological activities such as antiviral, antibacterial, anti-inflammatory, antiprotozoal, antidepressant, anticarcinogenic, antifungal, anthelmintic, DNA binding abilities etc. [1-6]. Quinoxaline derivatives are also used in electroluminescent devices, dyes, chemosensors, catalysts, waste water treatment, and in corrosion inhibition [7-13]. In view of the chemical and biological importance of the quinoxaline based metal complexes, we have synthesized Zn (II) and Cd(II) metal ion (M) complexes of the tridentate Schiff base ligand (L) 3-(2-(2-hydroxybenzylidene)hydrazinyl) quinoxalin-2(1H)-one(HSHQO) and investigated their interaction with Calf Thymus(CT) DNA by absorption spectroscopic studies.
Materials and Methods
The chemicals used were of reagent grade. Oxalic acid, o-phenylene diamine, zinc perchlorate, cadmium chloride, salicylic acid and solvents were purchased from Merck/Aldrich and were used as received. CT-DNA was purchased from Sigma. IR spectra were recorded on JASCO FTIR-5300 spectrometer in KBr pellets from 400 to 4000 cm-1. 1H NMR spectrum was recorded on Bruker 500 MHz spectrometer using DMSO d6 solvent. Electronic spectra were recorded on Shimadzu UV-3600 spectrometer. LCMS was recorded using Shimadzu mass spectrometer. Elemental analysis was carried out on FLASH Ea 1112 SERIES CHNS analyzer.
Synthesis of ligand (HSHQO)(L)
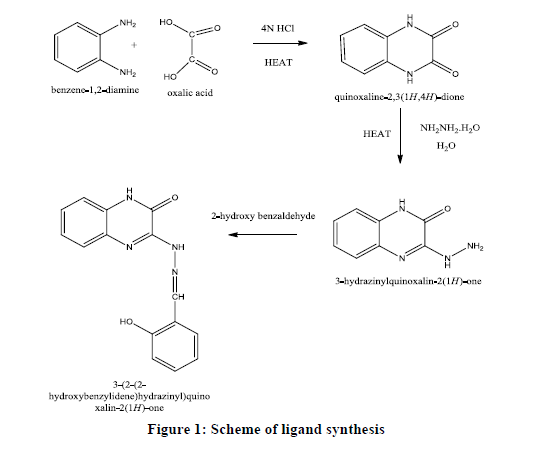
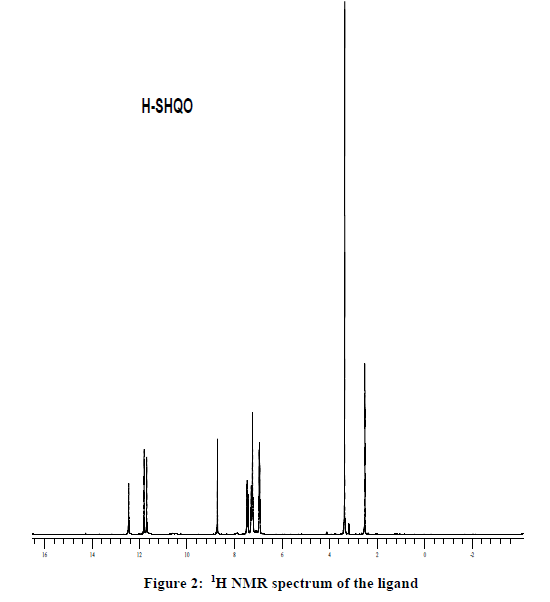
The ligand (HSHQO) was prepared by a reported three step procedure involving the synthesis of quinoxaline 2,3-dione and 3-hydrazino quinoxaline-2-one [14-16]. 1.76 g of 3-hydrazino quinoxaline-2-one was dissolved in hot methanol and 1.2 ml of salicylaldehyde was added to it. The mixture was refluxed for about 1 hr, the yellow product separated out was filtered hot, washed with methanol and n-hexane. The product was recrystallised from methanol. (MP 296ºC Yeild 78.57%) 1H NMR spectrum of the ligand was recorded in DMSO-d6: δ 12.44(1H, quinoxaline ring NH), δ 11.8 (1H, OH), δ 11.68 (1H, free NH), δ 8.71(1H, =C-H), δ 7.48-6.92 (8H, Ar-H) (Figures 1 and 2).
Synthesis of Zn(II) complex
Zn(II) perchlorate hydrate (0.1 mole 0.372 g) in methanol was ultrasonicated for 15 min. To the resulting solution, a methanolic suspension of (0.2 moles 0.56 g) ligand was added in small increments for about 20 min. A clear solution was obtained after each addition as the ligand dissolved completely in the presence of Zn(II) ion. The pH of the resulting mixture was adjusted to 7 using 1% methanolic ammonia solution and the solution was refluxed on a water bath for 2 hrs. The bright orange colored product separated was filtered hot and washed successively with hot methanol and finally with petroleum ether and dried over anhydrous calcium chloride. Elemental data: Found C=57.63%, H=3.61% and N=17.81%. Cal C=57.74%, H=3.55% and N=17.95%.
Synthesis of Cd(II) complex
CdCl2.H2O (0.1 mole 0.201 g) in water was heated and a hot methanolic suspension (0.2 moles 0.56 g) of ligand was added in small increments. The pH of the resulting mixture was adjusted to 7 using 1% methanolic ammonia solution and the solution was refluxed on a water bath for 2 hrs. The bright yellow colored product separated was filtered hot and washed successively with hot methanol and finally with petroleum ether and dried over anhydrous calcium chloride. Elemental data: Found C=53.48%, H=3.16% and N=16.36%. Cal C=53.70%, H=3.30% and N=16.70%.
DNA binding studies
Electronic absorption titrations were performed in KH2PO4 and K2HPO4 buffer using 10-4 molar solution of metal complexes in DMSO solution. The concentration of CT-DNA was determined from the absorption intensity at 260 nm and ε value (6600 M-1cm-1). The titrations were carried out by keeping the concentration of metal complex constant and varying the DNA concentration. The solution after each addition of DNA was incubated for 10 min before the absorption spectra were recorded. The intrinsic binding constant (Kb) was determined using the following equation.
[DNA]/(εa-εf)=[DNA]/(εb-εf)+1/Kb(εb-εf)
Where [DNA] is the concentration of DNA in base pairs, εa,εb and εf are apparent extinction coefficient, extinction coefficient for free metal complex and extinction coefficient for the metal complex in fully bound form respectively [13]. The Kb values were determined from the ratio of the slope to the intercept in the plots of [DNA]/(εb-εf) vs. [DNA].
Conclusion
Zn (II) and Cd(II) complexes of the ligand, HSHQO have been synthesised and characterized on the basis of various spectroanalytical data. HSHQO act as tridentate ligand with phenolic oxygen, imino nitrogen and ketonic oxygen as the donor centers. Both the metal ions formed 1:2 complexes (ML2) with the ligand. DNA binding studies revealed that the complexes exhibit significant ability to bind with CT-DNA through intercalation mode.
Acknowledgements
The authors are thankful to JNTUH, Hyderabad and Vasavi College of Engineering, Hyderabad for providing necessary facilities to carry out this work.
References
[1] N. Padmaja, M. Ravinder, P. Shilpa, J. Srihari J. Pharm. Chem., 2011, 5, 31-34.
[2] R. Mogili, M. Ravinder, K. Mamatha, N. Padmaja, N. Srihari, J. Pharm. Chem., 1, 2007, 35-39.
[3] A. Rogier, A. Smits, D. Herman D. Lim, A. Hanzer, O.P. Zuiderveld, E. Guaita, M. Adami, G. Coruzzi, R. Leurs, I.J.P. de Esch, J. Med. Chem., 2008, 51, 2457-2467.
[4] R. Mugdha, Suryawanshi, M. Vithal, Kulkarni, K.R. Mahadik, S.H. Bhosale, Arch. Appl. Sci. Res., 2011, 3, 380-391.
[5] R. Sarges, H.R. Howard, R.G. Browne, L.A. Lebel, P.A. Seymour, B. Kenneth, J. Med. Chem., 1990, 33, 2240-2254.
[6] P. Krishnamoorthy, P. Sathyadevi, A.H. Cowley, R.R. Butorac, N. Dharmaraj, Eur. J. Med. Chem., 2011, 46, 3376-3387.
[7] K.R. Justin Thomas, M. Velusamy, J.T. Lin, C.H. Chuen, Y.T. Tao, Chem. Mater., 2005, 17, 1861.
[8] A. Venkateswararao, P. Tyagi, K.R. Justin Thomas, P.W. Chen, K.C. Ho, Tetrahedron., 2014, 70, 6318-6327.
[9] X. Fang, G. Zhao, Y. Xiao, J. Xu, W. Yang, Tetrahedron. Lett., 2013, 54, 806-810.
[10] A. Zarrouk, A. Dafali, B. Hammouti, H. Zarrok, S. Boukhris, M. Zertoubi, Int. J. Electrochem. Sci., 2010, 5, 46-55.
[11] S. Adewuyi, Gangli, S. Zhang, W. Wang, P. Hao, W.H. Sun, N. Tang, Jianjun, J. Organomet. Chem., 2007, 692, 3532-3541.
[12] M. Sebatian, V. Arun, P.P. Robinson, A. Anna Varghese, R. Abraham, E. Suresh, K.K. Mohammed Yusuff, Polyhedron., 2010, 29, 3014-3020.
[13] S. Pushpa Latha, Int. Conf. Sci. Technol. Manag., 1391-1400.
[14] M. Alexandra Phillips, J. Chem. Soc., 1928, 2393-239.
[15] G.W.H. Cheeseman, M. Rafiq J. Chem. Soc., 1971, 453.
[16] P.V.A. Lakshmi, D.S. Rani, V. Jayatyagaraju, Asian. J. Chem., 1995, 7, 296-306.
[17] J. Dhanaraj Chellian, J. Johnson, Spectrochim. Acta., Part A, 2014, 127, 396-404.
[18] C. Justin Dhanaraj, J. Johnson, Spectrochim. Acta., Part A, 2014, 118, 624-631.
[19] A. Guha, J. Adhikary, T. Kumar Mandal, D. Das, Indian. J. Chem., Sect A, 2011, 50, 1463-1468.



